PAGE UNDER CORRECTION, UPDATE, AND OPTIMIZATION. PLEASE CHECK BACK IN TWO WEEKS
In this post, we discuss the diversity of obligate troglobitic, troglobiont, cave-dewlling, obligatory subterranean, and stygobitic animals in Brazil, collectively referred to here by the term troglobic (see discussion below). A comprehensive literature review was conducted, with a strong focus on the global context, and as of October 20, 2025, 866 troglobic species have been recorded in Brazil and 9,571 in World. Brazilian diversity span over 8 of the 11 Metazoa elegible phyla for troglobic: Porifera (2), Platyhelminthes (17), Nemertea (3), Annelida (10), Mollusca (29), Onychophora (1), Arthropoda (764) and Craniata/Actinopteri (40). Each species is presented in own card, featuring distribution map and images, when available. We also conducted cross-sectional analyses, examining the literature on the distribution of these species in various regions of the world, as well as their occurrence by state, municipality, and taxonomic group in Brazil.
This study chose to include, in the Brazilian totals and in some global totals, undescribed species. In the national case, more than 500 species cited in 30 articles as troglobitic and/or troglomorphic were compiled, many of them in a preliminary manner.
It is important to emphasize that this decision is highly unstable, as undescribed species may later prove to be synonyms of already described taxa, may not in fact be troglobitic after detailed analysis, may belong to a different group than originally cited, or may represent the same species cited in different articles. Additionally, there is the inherent conceptual difficulty in defining troglobiontism.
In this text, species are referred to by the names used in their original publications, and care will be taken to update this list should any of them be formally described in the future. Their global status will be corrected accordingly, and any species shown not to be truly troglobitic will be excluded. Details of these more than 500 undescribed species are provided in Section 6 of this work.
1 CONVENTIONS
For nomenclature, we use the notation ( z : y / ) x, which refers to x species in y genera of z families.
2 NOMENCLATURE AND DEFINITIONS OF TROGLOBICS
For the purposes of this text, based on Trajano & Bichuette (BOOK, 2006), we will use the following terminology for Metazoarian in caves: (1) troglobionts - obligatory subterranean, exclusively subterranean source populations; sink populations may be found in surface habitats; if obligate subterranean aquatic, we will call stygobionts; (2) troglophiles are subterranean source populations that maintain genetic connection with the epigean ones through individuals that disperse between these habitats; (3) trogloxenes are individuals regularly found in subterranean habitats but which must return periodically to the surface to complete their life cycle; they would be instances of source populations in epigean habitats, but using subterranean resources (the obligatory trogloxenes, all individuals are dependent on both subterranean and surface resources).
These terms sometimes overlap and blend, and in many contexts, they end up being inefficient in communication between different sources. Aiming to standardize the language used in this post, we have adopted the following terms to represent the different groups of obligatorily subterranean animals:
troglobic = obligatory subterranean, with exclusively subterranean source populations; sink populations may be found in surface habitats. Used here generically for terrestrial and freshwater species.
troglobiont = terrestrial troglobic, used in the text if necessary.
stygobitic = freshwater or amphibious troglobic, used in the text if necessary.
halotroglobic = marine troglobic, used in the text if necessary.
Troglobic are usually recognized by the presence of troglomorphisms - autapomorphies related to the subterranan selective regime. The commonest and most conspicuous troglomorphisms are reduction up to complete loss of visual structures and dark tegumentar pigmentation; other frequent troglomorphisms include elongation of barbels in fish and of appendices in arthropods, and reduction of wings in insects.
An excellent and important study on the troglophilic fauna of Brazil, which is neglected in conservation measures, is Duarte, Gallão & Bichuette (Biota Neotropica, 2025), citing 223 described troglophilic species, distributed across 51 orders and five phyla.
When discussing the concept of troglobic, four key questions arise for analysis. The first concerns whether this concept also applies to fungi and other organisms, such as protists, which are traditionally classified separately. The second question is defining which habitats qualify a potential troglobic as a true troglobic: anchialine caves, littoral interstitial habitats, deep phreatic and hyporheic of rivers, MSS (milieu souterrain superficiel, underground network of empty air-filled voids and cracks developing within multiple layers of rock fragments, generally covered by topsoil, SEE), epikarts and hypotelminorheic habitats can be? The third issue involves defining troglobic within well-established biological groups, exploring apomorphies (derived characteristics) and the phenomenon of troglomorphism, which refers to adaptations specific to subterranean environments. Finally, the fourth point questions to what extent the concept of troglobic can be applied to marine organisms, considering the unique features of aquatic environments and their caves. Culver, Deharveng, Bedos & Pipan (Diversity, 2021) makes interesting observations about some of these points.
EXCLUDED TAXA
The scope of this post is limited exclusively to obligatory subterranean freshwater and terrestrial species. A large number of references extend the term stygobiont to species inhabiting marine environments, especially anchialine systems. These are not considered here.
TAXONOMY OF EXCLUDED TAXA
Accordingly, we excluded as much data as possible on Stygiomysida (Mexico, Bermuda, among others), Thecostraca/Tantulocarida (Stygotantulus stocki Boxshall & Huys, 1989, which was reported from anchialine environments of the lava tubes in the Canary Island, parasitizing two families of Copepoda), Cumacea (subterranean species are collected from Bermudas, Bahamas and Jamaica), Tanaidacea (marine benthic habitats around the world, inhabiting anchialine and marine caves as halotroglobic in different regions of the world such as Bermuda and several islands of the South Pacific), Remipedia (25, Mexico, Belize, Caribbean, Canary Island, Australia), Stenopodida (1, Bahamas), Gebiida (1, Bahamas), Mictacea (a monotypic order with a halotroglobic, described for marine caves of Bermuda), Bochusacea (deep sea benthos, but includes two halotroglobic species of anchialine caves of the Bahamas) and Leptostraca (1, Providenciales caves in the Turks y Caicos), whose cave-restricted forms are entirely marine, as well as the marine forms of Anomura (1, Canary Is.), Amphipoda (Mexico, Bermuda, among others), Isopoda (Mexico, Bermuda, among others), Copepoda (Mexico, Bermuda, among others), Ostracoda (Mexico, Bermuda, among others), Mysida (Mexico, Bermuda, among others), Thermosbaenacea (includes freshwater troglobic and some halotroglobic worldwide), Caridea (Mexico, Bermuda, Honduras, Bahamas, among others, SEE) and Brachyura (no data).
All troglobic species in Branchipoda (Europe), Anaspidacea (Australia, New Zealand, Argentina and Chile), Astacidea (40, Papua New Guinea, USA, Mexico and Cuba), Spelaeogriphacea (W Brazil, South Africa and W Australia) and Bathynellacea (widely worldwide in subterranean habitats) are fully freshwaters.
Six infraorders of Decapoda include subterranean forms, with two of them consisting exclusively of marine species: Stenopodidae, represented by a species of the Macromaxillocarididae family from a anchialine cave in Bahamas; and Gebiidea, with a singles anchialine species of the genus Naushonia (Laomediidae). also for the Bahamas. One Anomura is a halotroglobic, in Canary Is. (Munidopsis polymorpha Koelbel, 1892).
ANCHIALINE SYSTEMS
An anchialine ecosystem is defined as 'a tidally-influenced subterranean estuary located within crevicular and cavernous karst and volcanic terrains that extends inland to the limit of seawater penetration' (Calderón-Gutiérrez et al., Regional Studies in Marine Science, 2017).
For geral data from species in anchialine caves, see Perez-Moreno et al. (International Journal of Speleology, 2016).
MEXICAN AND BERMUDA ANCHIALINE
Up to now, a total of 67 anchialine species have been recorded in anchialine caves from Mexico, all in Yucatan Peninsula, including organisms belonging to six phyla: Porifera, Annelida, Crustacea, Mollusca, Echinodermata, and Chordata. All 10 species of anchialine Porifera are all from Cozumel Island occurring so far, in only two caves: La Quebrada and Aerolito. One Annelida of the genus Speleonerilla (Nerillidae) from two cenotes in the Yucatan Peninsula is reported - however, they did not describe a new species due to the limited number of individuals available. Arthropoda represent the most diverse group in the anchialine caves of Mexico, with (23:36/)51 spp. that belong to 4 classes and 9 orders, with one endemic family (Anchialocarididae) and eight endemic genera (Mexicophria, Mayaweckelia, Tuluweckelia, Creaseriella, Yucatalana, Yagerocaris, Anchialocaris, Creasaria), plus 44 endemic species, in Remipedia (3), Melascostraca - Decapoda (16), Amphipoda (6), Mysida (3), Isopoda (7), Thermosbaenacea (1), Copepoda (12), and Ostracoda (3). Up to now, one species of anchialine Mollusca has been described from Mexico, the gastropod Teinostoma brankovitsi (Tornidae). Echinodermata anchialine fauna of Mexico includes Copidaster cavernicola, has been recorded so far from cenote Aerolito in Cozumel, and six undescribed species of asteroids and ophiuroids, all from cenote Aerolito that await formal description and always in sections next to connections to the sea due to marine nature from Echinodermata. Two species of Chordata (both Actinopteri), the blind cusk eel Typhlias pearsei (Dinematichthydae), and the blind eel Ophisternon infernale (Synbranchidae) occur in the anchialine caves of the Yucatan Peninsula, always in the freshwater layer (Alvarez et al., Mexican Fauna in the Anthropocene, 2023).
Five Mexican anchialine caves have more than 10 species, El Aerolito system has 100 (Calderón-Gutiérrez et al., Regional Studies in Marine Science, 2017). For Porifera in anchialine caves in Mexico, see (Goméz et al., Zootaxa, 2020). Alvarez et al. (Mexican Fauna in the Anthropocene, 2023) cites 67 anchialine species have been described in SE Mexico, 58 of which are endemic to the region. For aditional data for anchialine fauna distributed within a cave (Ox Bel Ha, SE Mexico), see Benítez et al. (Subterranean Biology, 2019).
The fauna inhabiting caves in the Walsingham Tract in Bermuda consists of 78 described species of cave-dwelling invertebrates, including 63 stygobionts and 15 stygophiles. Thus, it represents one of the world’s top hotspots of subterranean biodiversity. Of the anchialine fauna,
65 of the 78 species are endemic to Bermuda, while 66 of the 78 are crustaceans (Iliffe & Gutiérrez, Diversity, 2021). The text cites 63 stygobitics, but its table lists only 62, and excluding the questionable records of two Ciliophora, we conclude with 60 stygobic for the site: Annelida (3), Gastropoda (2), Acari (5), Copepoda (16), Ostracoda (13), Caridea (5), Isopoda (4), Amphipoda (5), Ingolfiellida (1), Tanaidacea (1), Mictacea (1), Cumacea (2), and Mysida (2).
EXCLUDED IN NATIONAL LISTS
Some anchialine species found in the revisions of national lists are rejected here: Cyathura univam (Isopoda, Anthuridae) and Metaniphargus venezuelanus (Amphipoda) in Venezuela [17]; Stygiomysis (2, Stygiomysida), Danielopolina mexicana Kornicker & Iliffe (Ostracoda), Agostocaris bozanici Kensley (Decapoda, Caridea), Triacanthoneus akumalensis Álvarez, Iliffe, González, & Villalobos (Decapoda, Caridea), Yagerocaris cozumel Kensley (Decapoda, Caridea), Barbouria yanezi Mejía, Zarza, & López (Decapoda, Caridea), Calliasmata nohochi Escobar-Briones, Camacho & Alcocer (Decapoda, Caridea), Procaris mexicana von Sternberg & Schotte (Decapoda, Procaridea) by [32], Xibalbanus (3, Remipedia), and Tulumella unidens Bowman & Iliffe, 1988 (Thermoasbaenacea) in Mexico (totaling 13 species, SEE | SEE).
In Venezuela, so far, only two endemic anchialine species, Cyathura univam Botosaneanu 1983 (Isopoda: Anthuridea) and Metaniphargus venezuelanus Stock & Botosaneanu 1983 (Amphipoda: Hadziidae), have been reported, and they were described from specimens collected in a cave not yet officially recorded by professional speleologists in Falcon state, specifically in the Mallorquines karst (Botosaneanu’s Cave, Morrocoy peninsula). These two species are the only representatives of their respective groups in South America and they were the result of the 1982 Amsterdam Expedition to the Venezuelan Islands and other localities on the mainland (Romero, Anartia, 2019).
REMIPEDIA
All (8:12/)28 spp. of Remipedia are halotroglobic anchialine (SEE), in Bahamas (7/13), Turks y Caicos (4/4), Mexico (1/3), Canary Is. (1/2), Australia (1/2), Republica Dominicana (1/2) and Cuba (1/2). Kaloketos, Lasionectes and Micropacteris occur only in Turks y Caicos. Angirasu, Cryptocorynetes, Godzilliognomus and Pleomothra occur only in Bahamas Godzillius in these both areas Other genera are Speleonectes (5, 4 in Bahamas, one in Cuba), Morlockia (4, Bahamas and Rep. Dominicana one each, 2 in Canary Is., Spain), Kumonga (1, NW Australia), and Xibalbanus (4, SE Mexico and Belize).
3 REFERENCES
[1] Gallão & Bichuette (ZooKeys, 2018), a primarily Brazilian troglobic list.
[2] White & Culver (Enciclopedia of Caves, 2019, 3th edition), general and generic diversity in caves worldwide.
[3] Freshwater Animal Diversity Assessment (Hydrobiologia, 2008), worldwide.
[4] Deharveng & Bedos (BOOK, chap. 7, 2018), worldwide.
[5] Palacios y Vargas (UMAE, 2013), for Mexico.
[6] Zampulo & Prous (Fauna Cavernicola do Brasil, 2022), for Brazil.
[7] Oliveira, L.F. (THESIS, 2024), for Africa.
[8] Jugovic, J. et al. (EJT, 2024), for Iran.
[9] Malek-Hosseini & Zamanai (Subterranean Biology, 2017), for Iran.
[10] Malek-Hosseini et al. (Subterranean Biology, 2022), for Iran.
[11] Almanaque Z/South America Cave Smaller Divesities, for Guyana, Suriname, French Guiana, Argentina, Chile, Paraguay and Uruguay.
[12] M. T. Guzik et al. (Invertebrate Systematics, 2010), for W Australia, unique data for this country.
[13] Díaz et al. (Biodiversidad de Cuba, cap. 15, 2015), for Cuba.
[14] Subterranean Fishes of the World (SFW) in December 15, 2024, worldwide.
[15] Furtado et al. (International Journal of Speleology, 2022) for Bolivia.
[16] Campos-Filho et al. (Nauplius, 2023) for Peru.
[17] Galán & Herrera (Boletín de la Sociedad Venezolan, Espeleologia-BSVE, 2006), for Venezuela.
[18] Almanaque Z/Mexican Cave Fauna in December 15, 2024 (SEE), for Mexico.
[19] Angarita-Sierra (CCE/2018 | Chapter Book, 2019) + Moreno-González, J.A. et al. (American Museum Novitates, 2023) for Colombia.
[20] Chatelliers et al. (Freshwater Biology, 2009), for Clitellata worldwide.
[21] Turbanov, I. et al. (PART 1, 2016 | PART 2, 2016), for former Sovietic Union.
[22] Peter Beron (Historia Naturalis Bulgarica, 2015), for SE Asia to New Guinea, including S China and S Japan.
[23] Nicholas, B.G. (The American Midland Naturalist, 1962), for Guatemala to Panama, and Caribbean except Cuba and Jamaica.
[24] Stewart B Peck (Canadian Journal of Zoology, 1999), for Jamaica.
[25] Culver et al. (Conservation Biology, 2021), for USA and Canada.
[26] Martínez, A. et al. (BOOK, 2016), for anchialine caves in Canary Is.
[27] Perez-Moreno, JL et al. (International Journal of Speleology, 2016), for anchialine caves worldwide.
[28] Golovatch, S. et al. (Invertzol, 2018), for European Russia.
[29] Sket et al. (Balkan Biodiversity, 2004), for Balkans in Europe.
[30] Zagmajster, M. et al. (Global Ecology and Biogeography, 2014), for European Pancrustacea.
[31] Pacheco, G. et al. (Insect Conservation and Diversity, 2020), for Guatemala.
[32] Palacios-Vargas et al. (EBM, 2015), detailed checklist from Mexico.
[33] Niemiller, M.L. et al. (Biodiversity and Conservation, 2025), detailed checklist from the USA and Canada.
4 TAXONOMIC SCOPE
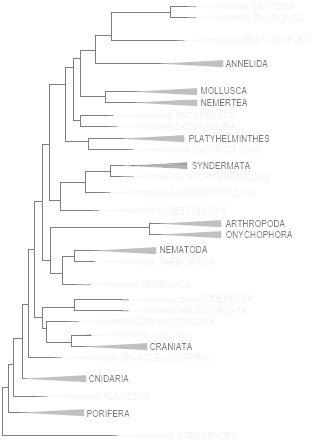
ALL 11 PHYLLA IN METAZOA WITH TROGLOBITS IN METAZOA PHYLOGENETIC TREE, AFTER LAUMER C.E. ET AL. (SEE, 2023 | SEE)
5 WORLD NUMBERS
How Many Species of Troglobites [troglobic] Are There? This is the title of an article by Culver & Holsinger (National Speleological Society, 1992), where they estimate there are between 50,000-100,000 troglobic species on the planet. Hobart M. King in Geology (SEE) cites a total of 7,700 spp.
We present below numbers from all regions around the world. However, it is important to highlight that these numbers are subject to various biases: many are poorly accurate, do not follow strong criteria regarding the concept of troglobic adopted, some are heavily outdated, much information is unavailable or not compiled, and several conflicts between these numbers can be pointed out. We use them here as reference points for compilation within the most reliable data available. We have extreme consistent data for former URRS (including Central Asia), Africa, Iran, SE Asia to New Guinea and Vanuatu, China, Canada/USA, Mexico, Guatemala to Panama, Caribbean (Jamaica and Cuba separately), Venezuela, Colombia, Ecuador, Peru, Bolivia and Brazil, and few consistent data for Europe (especially in Coleoptera and Diplopoda), Middle East except Iran, India Subcontinent, Japan, Australia, smaller Pacific islands except Vanuatu, New Zealand, Guianas and Cono Sur.
In October 20, 2025 our work believes that 9,571 troglobic documented in world, excluding 4 overlooping counts: 4,134 troglobic from Europe to Asiatic Russia, 84 in Middle East to Central Asia and Indian Subcontinent, 998 in E & SE Asia to New Guinea, 372 from Africa and Madagascar, 787 in Australia and Pacific Islands, and 3,197 in New World.

HOTSPOTS
A hotspots of subterranean biodiversity is a site with 25 or more troglobic, excluding deep soil sites or hyporheic sites unconnected with caves. There are 28 hotspots, that represent almost all subterranean biodiversity hotspots documented thus far at the world scale, in USA (5), Mexico (1), Brazil (3), Bermuda (1), Spain (3), France (4), Slovenia (2), Croatia (1), Bosnia & Hercegovina (1), Romania (1), China (1), Thailand (1), Vietnam (1), Indonesia (1) and Australia (2). Among these, only Brazil, Australia and Spain has non karstic ones (Culver, Deharveng, Bedos & Pipan, Diversity, 2021). Additionally, a new hotspot from Brazil was described in 2025: Padre Cave, Santana municipality, Bahia state (SEE).
If we consider only the systems with both troglobites and stygobites, they are reduced to 20: USA (3), Brazil (3), Spain (1), France (3), Slovenia (2), Croatia (1), Bosnia & Hercegovina (1), Romania (1), China (1), Thailand (1), Indonesia (1) and Australia (1).
If we consider only the systems with 8 or more troglobic in both groups (hyperhotspots), they are reduced to 13: USA (3), Brazil (1), Spain (1), France (2), Slovenia (2), Croatia (1), Bosnia & Hercegovina (1), Romania (1) and Indonesia (1).
In Brazil, Pedro Cassiano Cave (SEE) and Toca do Gonçalo Cave (SEE), both in Bahia state, are two cave systems with 20 to 25 troglobic.
OVERLAPPING SPECIES
At least 4 species occur in more than one area: Namanereis cavernicola Solís-Weiss & Espinasa, 1991 in the Caribbean and Mexico, Erpobdella absoloni Johansson, 1913 in Western Europe and the former USSR region (Georgia), Prietella phreatophila Carranza, 1954 in the USA and Mexico, and Onychiurus acuitlapanensis Palacios-Vargas & Deharveng, 1982 (Onychiuridae) from Venezuela and Guerrero state in Mexico (Galán & Herrera, BSVE, 2006).
EUROPE EXCEPT RUSSIA (3,852)
Although Europe is by far the region of the world with the greatest diversity of described troglobic forms, continent-wide syntheses by group are rare, and the totals reported for many groups are often based on data from specific regions. [2] mentions 5,000 species, although the unified data compiled here does not support this number. Croatia includes 299 troglobionts and 170 stygobitic taxa, including more 100 beetles (Gottstein, S. et al., Natura Croatica, 2002).
Europe (especially Balkans) shared with Brazil and Mexico the unique troglobic Porifera worldwide (1, in Balkans), shared with Brazil the unique Bivalvia worldwide (1, in Balkans), shared with Brazil and New Zealand the unique troglobic Nemertea (2, France, Switzerland, Germany, Bosnia and Herzegovina), shared with USA the unique troglobic Amphibia (1, Italy, Slovenia, Croatia, and Bosnia-Herzegovina), shared with Colombia (possibly) the unique Nematoda worldwide (7, Belgium, Austria, Hungary, Slovenia, Romania), and has exclusivity among Temnocephalida (15), freshwater troglobic Sabellida (1, Italy, Slovenia, Croatia, Bosnia & Hercegovina) and Cnidaria worldwide (1, Slovenia, Croatia, Bosnia & Hercegovina).
Numbers for Europe except Russia and post-Sovietic state includes (for detailed numbers, check each group's topic): Porifera (1), Cnidaria (1), Platyhelminthes (112), Nemertea (2), Nematoda (7), Gastropoda (368), Bivalvia (1), Annelida (103, being one Phyllodocida, two Sabelliida and 100 Clitellata), Acari (8), Araneae (194), Pseudoscorpiona (132), Opiliona (21), Palpigrada (23), Scorpiona (1), Symphyla (2), Diplopoda (191), Chilopoda (55), Ostracoda (114), Copepoda (547), Branchiopoda (7, Cladocera), Malacostraca (900), Colembola (338), Diplura (110), Coleoptera (607), Dermaptera (1, from Canary Is.), Zygentoma (1), Diptera (2), Actinopteri (2) and Amphibia (1), totalling 3,826 troglobic in Europe.
Europa includes stygobitic in Ostracoda (114), Copepoda (547), Branchiopoda (7, Cladocera) and Malacostraca (900, being Isopoda 337, Amphipoda 438, Bathynellacea 106, Thermosbaenacea 2, Mysida 3, Decapoda 16), these by Zagmajster, M. et al. (Global Ecology and Biogeography, 2014). No data for remaining Crustacea.
Troglobic Onychophora, Symphyla, and Pauropoda are fully unknown in Europe in any form.
The Canary Islands are the richest volcanic region in the world in subterranean adapted fauna (294 troglobionts and stygobionts), followed by the Hawaiian Islands and the Undara Cave in Australia. Most of the subterranean adapted aquatic fauna from the Canary Islands is restricted to the anchialine environments in La Corona lava tube in Lanzarote, while the oligohaline stygobiont fauna, usually found in groundwater or interstitial freshwaters, is scarcer and represented by a few species of amphipods, copepods, and a single polychaete annelid recorded from Fuerteventura. In fact, Cueva de Felipe Reventón (37) and Cueva del Viento-Sobrado (36), both in Tenerife, are the second and third caves with the greatest number of troglobiont species in the world ranking, while La Corona lava tube, in Lanzarote, is the fourth richest in anchialine species (Nuñez, J., Subterranean Biology, 2020).
AFRICA AND MADAGASCAR (372)
[7] lists the troglofauna of Africa, with 396 spp., excluding Actinopteri, but presents some inconsistencies in its numbers. Although it mentions six phyla, only five are listed (page 107). The sum of the clades in Arthropoda totals 344 spp., not 347 as indicated. Three Platyhelminthes are cited, but the list includes six (three Trepaxonemata and three Turbellaria). The sum of Harpacticoida and Cyclopoida results in 59, exceeding the 50 species of Copepoda mentioned.
In the graphs on page 108, three Tricladida and three Neoophora are cited. For the Platyhelminthes, we assume that all references refer to three specific species, and the ambiguous repetitions stem from the repeated inclusion of a group at different hierarchical levels. Chilopoda (2) appears as 'Chilopoda' (1), Scolopendromorpha (1), and Lithobiomorpha (1) in the graphs, but here we adopt Scolopendromorpha (1) and Lithobiomorpha (1) to maintain the numbers as per the original list. 'Blattaria' (4) and 'Blattodea' (1) are shown separately in the graphs, but here we treat them as Blattodea (5). The category Pseudoscorpiones is repeated twice, totaling six species. Amphipoda is mentioned three times, including Bogidellidae, with a total of 72, but we adopt the 69 species initially listed. The graphs indicate 36 Harpacticoidea, 1 Crangonyctoidea, 1 'Copepoda', 1 Calanoidea, and 23 Cyclopoidae, with an actual total of 62; this number is accepted, excluding 1 Copepoda due to lack of details. The mention of 'Malacostraca' (2) is rejected due to insufficient information. The reference to 'Myriapoda' (1) is also rejected, as well as the mention of 'Crustacea' (3) and 'Maxillopoda' (14) due to the lack of details. Finally, the graphs indicate that 'Entognatha' (2) refers to Diplura (2), and Dermaptera refer a species from Canaray, and treated here as Europe.
Based on these considerations, the direct filtering of [7] results in 356 troglobites in Africa, distributed among the following groups: Platyhelminthes (3), Annelida (2, Clitellata), Mollusca (16), Onychophora (1, in South Africa), Arachnida (32, Trombidiformes 1, Schizomida 1, Pseudoscorpiones 6, Amblypigy 1, Opiliones 2, Araneae 21), Diplopoda (5, 1 Spirostreptida, 1 Chordeumatida, 3 Polydesmida), Chilopoda (2), Ostracoda (4, Podocopida), Copepoda (61, Harpacticoida 36, Cyclopoida 23, Calanoidea 1, Crangonyctoidea 1), Malacostraca (188, Isopoda 71, Amphipoda 69, Bathynellacea 27, Decapoda 15, Thermosbaenacea 5, Spelaeogriphacea 1), Diplura (2), Collembola (5, Poduromorpha 2, Entomobryomorpha 3) and Insecta (36, Coleoptera 23, Diptera 3, Blattaria 5, Orthoptera 1, Hemiptera 1, Hymenoptera 1, Lepidoptera 1).
Also by [7], 32 african countries has troglobic, mainly in Morocco (92), Madagascar (64), Algeria (45), South Africa (34) and Namíbia (16). Addionally, five countries in Africa has 11 troglobic Actinopteri: Cameroon (1:1/1), Namibia (1:1/1), Somalia (2:3/3), D.R. Congo (1:1/1) and Madagascar (2:2/5), by [14], Sendra et al. (Zoological Journal of Linnean Society, 2021) cites 4 Diplura (two more), two Scorpion is cited for Madagascar (SEE), and [20] cites one more Haplotaxidae from Guinea — 14 added species.
Thus, Africa has 372 troglobic species. For details among Malacostraca, Copepoda and Ostracoda from Africa, see Raoul, TK et al (Crustaceana, 2012). Aziza cave in SE Morocco includes remarkable 22 troglobiotic and 4 stygobitic species, mainly in Coleoptera (5), Araneae (4), Entomobryomorpha (3) and Isopoda (2), by Moutaouakil, S. et al. (Subterranean Biology, 2024).
FORMER USSR EXCEPT BALTIC COUNTRIES (276)
[21] lists 269 spp. from former USSR except Baltic countries (EX-USSR/SS), in Russia (150, Tricladida 3, Annelida 2, Gastropoda 9, Bivalvia 1, Opiliones 5, Pseudoscorpiona 3, Acari 5, Chilopoda 1, Diplopoda 5, Ostracoda 4, Copepoda 19, Bathynellacea 6, Amphipoda 22, Isopoda 19, Decapoda 1, Collembola 21, Diplura 6, Coleoptera 18, mainly in Crimea and Caucasus), Abkhazia (68, Tricladida 1, Annelida 4, Gastropoda 12, Bivalvia 2, Opiliones 3, Palpigrada 1, Chilopoda 1, Diplopoda 4, Copepoda 5, Amphipoda 12, Isopoda 3, Decapoda 4, Collembola 6, Coleoptera 10), Georgia (35, Annelida 3, Gastropoda 4, Bivalvia 1, Opiliones 1, Pseudoscorpiona 2, Diplopoda 1, Ostracoda 1, Copepoda 7, Bathynellacea 1, Amphipoda 2, Isopoda 3, Decapoda 2, Collembola 2, Diplura 1, Coleoptera 4), Ukraine (13, Bathynellacea 3, Gastropoda 1, Amphipoda 1, Collembola 4, Coleoptera 3, Copepoda 1), Armenia (1, Bivalvia), Azerbaijan (3, Amphipoda 2, Collembola 2), Kazakhstan (2, Amphipoda), Uzbekhistan (5, Copepoda, Annelida, Bathynellacea, Isopoda and Collembola one each), Kyrgystan (5, Bathynellacea 4, Isopoda 1), and Turkmenisthan (4, Isopoda 2, Gastropoda and Coleoptera one each). Five of these species are shared: two Russia/Abkhazia (Conulopolita cavatica and Nemaspela abchasica), 1 Armenia/Georgia (Euglessa subterranea), two in Russia/Abkhazia/Georgia (Bryocamptus innominatus, Echinocamptus georgevitchi). Belarus, Moldova and Tajikistan does no has troglobic.
The total number of entries in this list is 287 spp. After excluding 7 redundant repetitions (12 repeated entries involving 5 species), we arrive at 280 validly cited species so far. Additionally, two more troglobic are known in Turkmenistan: Turkmenocampa mirabilis Sendra & Stoev, 2017 (Diplura), found in Kaptarhana cave, in Lebap province (Sendra, A. et al., Subterranean Biology, 2017), and Paracobitis starostini Parin, 1983 (Actinopteri, Nemacheilidae), by [14].
Thus, troglobic in EX-USSR/SS are 282, being Tricladida (4), Annelida (10, one shared with W Europa), Gastropoda (27), Bivalvia (3), Acari (5), Pseudoscorpiona (5), Opiliones (8), Palpigrada (1), Chilopoda (2), Diplopoda (11), Ostracoda (5), Copepoda (30), Bathynellacea (15), Amphipoda (41), Decapoda (7), Isopoda (29), Diplura (7), Collembola (35), Coleoptera (36) and Actinopteri (1).
MIDDLE EAST EXCEPT IRAN PLUS AFGANISTAHN (13)
Middle East and Afganisthan no has unified checklist of troglobic available. At least 13 troglobic: 1 in Scorpiona (Israel, Akrav israchanani, SEE), 1 in Diplura (Sendra et al., Zoological Journal of Linnean Society, 2021, in Lebanon), three Namanereis (Annelida/Nereididae), one in Oman and two in Socotra [2], two Clitellata in Oman [20], and six Actinopteri, in Iraq (2:3/3) and Oman (1:1/3), by [14].
IRAN (49)
Iran includes 51 troglobic by [8], being 46 aquatic and five terrestrial. However, we were only able to identify 48 of them in this post: 24 Amphipoda, 12 Copepoda, 3 Isopoda [8], Araneae and Diplopoda one each [9], one Clitellata (Erpobdella borisi Cichocka & Bielecki, 2015), one Gastropoda and (2:2/)5 Actinopteri [14]. Addtionally, one Hymenoptera was collected in latter (Yavnella laventa, SEE).
INDIAN SUBCONTINENT (22)
Indian Subcontinent (Pakistan, India, Bangladesh, Nepal, Butan and Maldivas) no has unified checklist of troglobic available. 21 troglobic Actinopteri are known from this regions, in India (7:10/21), by [14], and one Diplura, also in India (Sendra et al., Zoological Journal of Linnean Society, 2021).
JAPAN (93)
Japan no has unified checklist of troglobic available. Nearly 200 spp. of troglobic are known from the groundwaters of Japan. Most of these troglobic species, 16 of 77 genera, and what is more, 4 of 47 families are endemic to Japan. Uchidastygacaridae, Nipponacaridae, and Kantacaridae are endemic acaridan families of Japan (Matsumoto, Kôichi, International Journal of Speleology, 1976). Two Clitellata [20], two Staphylinidae (Hlavac et al., Subterranean Biology, 2006), 80 Coleoptera in Shikoku (SEE), and one Diplura (Sendra et al., Zoological Journal of Linnean Society, 2021) are troglobic in Japan. (1:1/)5 troglobic Actinoperi, all in Luciogobius (Gobiidae), are known from Japan [14].
KOREA (11)
11 troglobic species are recorded from the Korean Peninsula, all occurring in South Korea: Uenohadesina styx Smetana, 2000 (Staphylinidae, Hlavac et al., Subterranean Biology, 2006), seven Spelaeochthonius (Pseudotyrannochthoniidae, SEE), and 3 Pseudocrangonyx (Pseudocrangonyctidae, SEE). Byung-Woo Kim et al. (Korean J. Environ. Biol., 2004) listed 257 cave-dwelling species from the Korean Peninsula; however, the study does not clearly distinguish troglobic from non-troglobic species, which makes it of limited use for assessing the region’s troglobic fauna.
CHINA (710)
We considere here 710 troglobic in China, in Gastropoda (2), Araneae (104), Pseudoscorpiona (40), Chilopoda (1), Diplopoda (114), Copepoda (23), Bathynellacea (1), Amphipoda (26), Decapoda (23), Isopoda (18), Collembola (42), Orthoptera (40), Coleoptera (159), Hemiptera (1), Diptera (2), these by [2], Annelida (2) by [2], Diplura (5) by Sendra et al. (Zoological Journal of Linnean Society, 2021), and Actinopteri (107 in three families), by [14], near a half are fishes, beetles and spiders.
[2] cites one cave-dwelling Anura in China, however is fully rejected here as troglobic, being excludes in this count.
SE ASIA FROM MYANMAR TO NEW GUINEA, TIMOR AND PHILIPPINES (184)
[22] lists all the non-Actinopteri troglobionts from SE Asia to New Guinea. Their list contains inconsistencies between the manual counts and the numbers provided in the abstract: 16 fewer species are counted in SE Asia and 6 fewer in New Guinea. Considering only the 11 politically recognized SE Asian countries (Myanmar, Thailand, Laos, Vietnam, Cambodia, Malaysia, Cingapure, Indonesia, Philippines, Brunei, East Timor, and Papua New Guinea), excluding China and Japan members, we total 146 troglobic species, being Phyllodocida (1, Namanereis from Papua New Guinea), Gastropoda (1), Araneae (13), Pseudoscorpiones (4), Scorpiona (2, Troglokhammouanus steineri in Laos and Vietbocap canhi in Vietnam), Thelyphonida (1, Typopeltis magnificus Haupt, from Laos), Opiliones (3), Acari (4), Diplopoda (15), Amphipoda (3), Isopoda (22), Decapoda (22), Diplura (2), Collembola (23), Orthoptera (1), Blattaria (8), Coleoptera (18), Hymenoptera (1, Leptogenys khammouanensis) and Diptera (2).
Additionally, are documented from this regions 38 new troglobic: two scorpions in Indonesia and one in Malaysia (SEE), Orphnaecus pellitus Simon, 1892 (Theraphosidae, Philippines, SEE), two in Diplura (Myanmar-1 and Papua New Guinea-1, in Sendra et al., Zoological Journal of Linnean Society, 2021), three in Thermosbaenacea (Theosbaena, in Thailand-2 and Cambodia-1, see discussion in Thermosbaenacea topic), and 29 in Actinopteri (Myanmar-1:1/1, Thailand-4:5/9, Laos-2:3/3, Vietnan-3:6/7, Malaysia-1:1/1, Philippines-2:2/3, Indonesia-3:3/3 and Papua New Guinea-1:1/2), these by [14].
By country/or region: Myanmar (4), Thailand (49), Laos (17), Cambodia (2), Vietnan (24), Philippines (15), continental Malaysia (4), Malaysian Sarawak (19, including one scorpion), Malaysian Sabah (1), Sumatra and adjacent Batu (6), Java and adjacent Sumba (6, including one scorpion), Indonesian Kalimatan (4), Sulawesi (8, including one scorpion), Moluccas (1) and New Guinea (25).
Thus, SE Asia includes 184 troglobic species.
Two species that were supposedly identified as probable troglobic species in [2] are not recognized as such in [22]: one Temnocephalida and Leiobdella jawarerensis Richardson, 1974 (Clitellate/Haemadipsidae), both for Papua New Guinea. Towakkalak cave in Sulawesi has 36 spp. of troglobic (SEE), but non counted here.
Numerous data on the troglofauna of Southeast Asia can be found for the Mo So Cave and the Hon Chong Karst of Vietnam (Diversity, 2023) and Towakkalak System in Sulawesi, Indonesia (Diversity, 2021), including information that may complement — or even contradict — the findings presented in this text. Hang Mo So has 27 troglobic, including many still undescribed. An additional 40 troglobic species are known from other caves of Mekong Delta Limestones of Vietnam. There are 10 stygobic and 26 troglobionts that are known from the Towakkalak System.
AUSTRALIA (697)
Australia no has unified checklist of troglobic available, and the lack of precision in the numbers from Australia is absurdly complicating and confusing. [2] cites troglobic Amphipoda (144), Palpigrada (1) and Scopiona (2, SEE | SEE). We combined these numbers with the following numbers from [12]: Aracnida (101, for uniquely didactic effect, all assignated for Araneae), Myriapoda (19, for uniquely didactic effect, all assignated for Diplopoda), Hexapoda (164, for uniquely didactic effect, all assignated for Coleoptera), 'terrestrial Crustacea' (45, for uniquely didactic effect, all assignated for Isopoda), Gastropoda (4), Ostracoda (74), Copepoda (83), Bathynellaceae (1) and Isopoda (49), who discussed the troglofauna of Western Australia. We excluded from this second work the imprecise placements or defased numers for some groups: 'other Stygofauna' (30).
Additionally for troglobic, there are Hanseniella magna Scheller in Tasmania (Symphyla, SEE), one more Diplura (Sendra et al., Zoological Journal of Linnean Society, 2021), one Clitellata [20], Namanereis pilbarensis Glasby, Fiege & Van Damme, 2014 (Nereididae) in Pilbara region in Australia (SEE), (2:2/)3 Actinopteri (in Milyeringidae and Synbranchidae) in N Australia [14], Halosbaena tulki Poore & Humphreys, 1992 is Western Australia (Wikipedia), and Anaspidacea (3, Wikipedia).
In these fragmented and imprecise numbers, we estimate the diversity of troglobites in Australia at 779 spp.
OCEANIA EXCEPT AUSTRALIA AND NEW GUINEA (8)
Pacific region except Papua New Guinea and New Zealand no has unified checklist of troglobic available. At least one species of troglobic Nemertea is known in New Zealand, Exosphaeroides quirosi Jaume et Queinnec, 2007 (Sphaeromatidae, Isopoda) for Vanuatu [22], Phreodrilus subterraneus Beddard, 1891 (Clitellata/Phreodrilidae) also in New Zealand [20], one Namanereis (Annelida/Nereididae) in New Zealand and Fiji [2], ona Gastropoda in New Zealand [2, Hydrophrea], one Actinopteri from Guam (1:1/1), by [14], and two Anaspidacea in New Zealand (Stygocarella pleotelson Schminke, 1980 and Stygocaris townsendi Morimoto, 1977, SEE).
Stewart, Robertson & Kasper (N.Z. Speleological Bulletin, 2023) discuss cave fauna in New Zealand in a highly allegorical manner, which makes the data difficult to use and, therefore, not considered here.
USA/CANADA (1,473)
[33] lists all troglobic from the USA and Canada, which includes Alaska and Hawaii, with (124:288/)1,460 spp., representing six phyla: Platyhelminthes (34), Syndermata (1), Annelida (4:8/22), Gastropoda (39), Acari (32), Araneae (121), Opiliones (46), Pseudoscorpiones (153), Schizomida (2), Scorpiones (1), Copepods (18), Ostracoda (22), Bathynellacea (23), Thermosbaenacea (1), Amphipoda (192), Isopoda (118), Decapoda (44), Chilopoda (4), Diplopoda (140), Collembola (101), Diplura (10), Coleoptera (284), Dermaptera (1), Diptera (3), Hemiptera (10), Lepidoptera (1), Orthoptera (6), Zygentoma (8), Actinopterygii (10) and Amphibia (12).
Some genera represent remarkable subterranean radiations in the USA, such as Pseudophthalmus (147, Coleoptera), Stygobromus (139, Amphipoda), Pseudotremia (62, Diplopoda), Caecidotea (56, Isopoda), Cicurina (52, Araneae), Tyrannochthonius (36, Pseudoscorpiona), Pygmarrhopalites (31, Collembola), and Batrisodes (30, Coleoptera). Together, these 8 genera account for 553 troglobic species in the USA, representing nearly 2/5 of the national diversity.
Only 11 spp. occur as troglobic in Canada: one Rhagidiidae, three Araneae (Linyphiidae), one Collembola/Entomobryidae, one Collembola/Arrhopalitidae, four Amphipoda/Stygobromus, and one Isopoda/Asellidae. In Hawaii, there are 60: two Rhagidiidae, five Araneae (two Linyphiidae, two Lycosidae, one Ochyroceratidae), five Pseudoscorpiones/Chthoniidae, five Collembola/Entomobryidae, one Collembola/Neanuridae, four Coleoptera/Carabidae, two Diptera, and all troglobic Hemiptera (10), Dermaptera (1), Lepidoptera (1), and Orthoptera (6) from the USA, ten Amphipoda, four Isopoda/Philosciidae, two Atyidae, and two Procarididae. Only two troglobic occur in Alaska: one Amphipoda and one Bathynellacea.
Additionally, Salishbaena kootenai Wagner & Reid, 2021 (Thermosbaenacea, SEE), and 12 more Actinoperi [14] are known as troglobic from region. Thus, USA (including Hawaii and Alaska) and Canada includes 1,473 troglobic.
BRAZIL/USA COMPARATION
Neither Brazil nor the USA have troglobic in Cnidaria.
Troglobic lineages in Brazil unknown as troglobic in USA are Porifera, Nemertea, Onychophora, Bivalvia, Pauropoda, Symphyla, Blattodea and Hymenoptera.
Troglobic lineages in USA unknown as troglobic in Brazil are Platyhelminthes/Alloeocoela (1/1), Platyhelminthes/Cestoda (1/1), Platyhelminthes/Trematoda (1/1), Syndermata/Acanthocephala (1/1), Nematoda (2/2), Thermosbaenacea (1/1), Lepidoptera (1/1), and Amphibia (1:2/12).
At commom groups, Brazil and USA are fully tied in Dermaptera,Orthoptera, and Sarcoptiformes.
At commom groups, USA surpasses the Brazil in the following groups (US excedents in parentesis): Coleoptera (3F, ≤19G, 196s), Amphipoda (6F, 19G, 169s), Pseudoscorpiona (2F, ≤20G, 105s), Diplopoda (1G, 46s), Araneae (≤1G, 40s), Decapoda (2F, 6G, 40s), Bathynellacea (1F, 6G, 22s), Ostracoda (1F, 13G, 21s), Trombidiformes (3F, ≤12G, 20s), Tricladida (14s), Copepoda (≤3F, ≤5G, 13s), Annelida (≤2F, ≤6G, 12s), Gastropoda (≤7G, 10s), Opiliones (3s), Mesostigmata (≤1F, ≤1G, 2s), Schizomida (1F, 1G, 1s), Collembola (≤3G), Diplura (≤1G), and Zygentoma (1s).
At commom groups, Brazil surpasses the USA in the following groups at all levels: Chilopoda, Scorpiona, Palpigrada, Hemiptera, Isopoda and Actinopteri.
MEXICO (523)
Mexico has 523 spp. of troglobic in the following groups [5]: Porifera (1, Legendre et al., Subterranean Biology, 2023, undescribed), Platyhelminthes (2:2/6, 4 Continenticola, 2 Cavernicola), Annelida (3/5, Errantia-1, Oligochaeta-2, Branchibdellida-2), Gastropoda (3:5/9), Trombidiformes (2), Pseudoscorpiona (5:40), Araneae (11:59), Opiliones (4:17, SEE), Amblypygi (11, all Phrynidae), Schizomida (23, Hubbardiidae-14, Protoschizomidae-9), Ricinulei (11, all in Pseudocellus), Scorpionida (4:8/13), Diplopoda (15:/73, at six orders), Chilopoda (5, Lithobiidae-2, Newportia-3), Copepoda (5:8/12), Ostracoda (2:2/3), Amphipoda (2:22 spp.), Mysida (2:3/5), Decapoda (20: 2:10 shrimps, 1:5 crayfishes, and 2:5 crabs), Isopoda (11/52), Collembola (9:42), Diplura (4/8, Litocampa-2, Juxtlacampa-2, Oncinocampa-1, Tachycampa-3, Sendra et al., Zoological Journal of Linnean Society, 2021), Zygentoma (3/13), Orthoptera (6, all Phalangospsidae), Coleoptera (44, Carabidae-30, Dytiscidae-1, Histeridae-4, Leiodidae-8, Ptinidae-1), Hemiptera (3, all Fulguromorpha), and Actinopteri (7:7/18 spp. at four orders).
Tulumella unidens Bowman & Iliffe, 1988 (Thermosbaenacea), Danielopolina mexicana Kornicker & Iliffe (Ostracoda), Xibalbanus (3), Stygiomysis (2), and 7 Caridea was excluded due to its anchialine nature, which falls outside the scope of this article.
Mexico no has described troglobic in Nematoda, Nemertea, Onychophora, Mollusca/Bivalvia, Bathynellacea, Spelaeogriphacea, Blattodea, Dermaptera, Diptera, Hymenoptera, Pauropoda, Symphyla, Palpigradi or Amphibia. Brazil has more species than Mexico in all groups except in Copepoda, Ostracoda, Amphipoda, Mysida, Decapoda, Zygentoma, Coleoptera, Diplopoda, Pseudoscorpionida, Araneae, Amblypygi, Schizomida, Ricinulei and Scorpionida, all in Arthropoda.
Hlavac et al. (Subterranean Biology, 2006) cite Stenophloeus reddelli Herman, 1969 as troglobic in Mexico; however, it is rejected here based on [5].
AMERICA CENTRAL (GUATEMALA TO PANAMA, 20)
The only consistent reference on the troglobic fauna of southern Mexico, America Central, and the Caribbean is [23], who lists 170 spp. for the vast region. Currently, Mexico, Cuba, and Jamaica have more recent references and are treated independently. Therefore, we use these references only for the region from Guatemala to Panama and the West Indies, excluding Cuba and Jamaica.
Based on [23], only eight troglobic occur from Guatemala to Panama, six valid nowdays (Pselliodes harveyi Chamberlin, 1942 from Panama and Pselliophora cavincola Chamberlin, 1918 from Trinidad under Sphendononema guildingii Newport, 1845, SEE), namely: Otostigmus cooperi Chamberlin, 1942 (Chilopoda, Panama), Calymmodesmus inquinatus Causey, 1960 (Diplopoda, Guatemala), Lepidocyrtus usitatus Folsom, 1927 (Collembola, Panama), Ptomaphagus giaquintoi Jeannel, 1936 (Coleoptera, Alta Verapaz, Guatemala), Trombicula cavernarum Ewing,, 1933 (Trombidiformes, Panama) and T. trifurca Ewing, 1933 (Trombidiformes, Panama).
Additonally, troglobic added includes three crabs in the genus Typhlopseudothelphusa (Pseudothelphusidae, two in Guatemala and one in Belize), one Diplura in Guatemala (Sendra et al., Zoological Journal of Linnean Society, 2021), one Actinopteri from Belize, Rhamdia typhla Greenfield, Greenfield, and Woods, 1982, in Heptapteridae [14] and nine from Gruta de Lanquín in Guatemala [31 | Isopoda-2, Platyhelminthes-2, Araneae-1, Pseudoscorpiones-1, Opiliones-1, Polydesmida-1, Zygentoma-1].
For these numbers, America Central has 20 documented troglobic. Deleva, S. et al. (Subterranean Biology, 2023) discusses the cavernicolous fauna of Costa Rica and confirms that there are no troglobic species in the country, only strong candidates are Pseudothelphusa puntarenas Hobbs 1991 (Decapoda), various specimens of Trogolaphysa (Collembola) and some Acari (Rhagidiidae) from Barra Honda.
CUBA (130)
[13] cites 112 troglobic in Cuba, all Arthropoda: Acari (12), Scorpiones (1), Pseudoscorpiones (3), Amblypygi (1), Schizomida (5), Ricinulei (4), Opiliones (4), Araneae (7), Chilopoda (2), Ostracoda (5), Copepoda (18), Mysidacea (5), Isopoda (7), Decapoda (13), Amphipoda (3), Zygentoma (3), Collembola (7), Orthoptera (4), Blattoidea (1) and Coleoptera (6). Remipedia (1) excluded.
Additionally, one Palpigrada [2], 1 more Scorpiona (SEE), one Thermosbaenacea (Tethysbaena vinabayesi Wagner, 1994, see discussion in Thermosbaenacea topic), 5 Clitellata [20], two Chilopoda (see topic about Chilopoda) and nine Lucifuga (Bythitidae, Ophidiiformes, Actinopteri) are reported [14].
Thus, Cuba has 130 troglobic species in our survey.
JAMAICA (42)
Troglobic in Jamaica contains 43 spp. troglobic, being 27 of troglobionts in Onychophora (Speleoperipatus spelaeus Peck, 1975), Araneae (9), Opiliones (2), Pseudoscorpionida (4), Schizomida (1), Isopoda (1), Collembola (2), Blattodea (1), Hemiptera (2) and Coleoptera (4), and 16 stygobitics, in Tricladida (1), Ostracoda (3), Mysida (2), Thermosbaenacea (1), Decapoda (2), Amphipoda (4) and Isopoda (3), by [24]. Thermosbaenacea record is excluded here due to inconclusive status relative to the literature.
CARIBBEAN EXCEPT CUBA AND JAMAICA PLUS BERMUDA (27)
The only consistent reference on the troglobic fauna of southern Mexico, America Central, and the Caribbean is [23], who lists 170 spp. for the vast region. Currently, Mexico, Cuba, and Jamaica have more recent references and are treated independently. Therefore, we use these references only for the region from Guatemala to Panama and the West Indies, excluding Cuba and Jamaica.
[23] lists nine troglobic for this area, namely: Porcellionides minutissimus Van Name, 1936 (Isopoda, Bahamas), Bogidiella bredini Shoemaker, 1959 (Amphipoda, Barbuda), Metaniphargus beattyi Shoemaker, 1942 (Amphipoda, Virgin Islands), M. nicholsoni Shoemaker, 1959 (Amphipoda, Barbuda), Typhlatya monae Chace, 1954 (Decapoda Caridea, Puerto Rico), Maymena bruneti Gertsch, 1960 (Araneae, Trinidad), Stygiomysis holthuisi Gordon, 1960 (Mysida, St. Marteen), Calycuoniscus spinosus Collinge, 1917 (Isopoda, Trinidad, West Indies) and Philoscia bermudensis Dahl, 1892 (Isopoda, Walsingham Cave, Bermuda).
Additionally, 44 more troglobic are confirmed for this area: two Scorpiona (Heteronebo clareae Armas, 2001 in Bahamas, Cazierius ciguayo Teruel, Jiménez & Santos, 2021 and C. cayacoa Teruel, Jiménez & Santos, 2021 in Dominican Republic, SEE), Newportia stoevi Schileyko, 2013 (Chilopoda, Puerto Rico, SEE), six Namanereis (Annelida, Nereididae, one shared with Mexico), Phallodriloides macmasterae Erséus, 1986 (Tubificae) in Bermuda [20], Dero haitiensis Dumnicka, 1986 and Spirospermoides stocki Dumnicka, 1983 (both Tubificidae) in Haiti and adjacent islands [20], one Thermosbaenacea in Puerto Rico (Tethysbaena colubrae, see discussion in Thermosbaenacea topic), three Actinopteri, Rhamdia urichi Mees, 1974 (Heptapteridae), from Trinidad and Tobago, and two Lucifuga (Bythitidae) from Bahamas [14], and one Pseudoscorpiona from Aruba (Pseudochthonius arubensis Wagenaar-Hummelinck, SEE).
Thus, in West Indies except Jamaica and Cuba are known 27 troglobics.
GUIANAS (0)
[11] cites possibly Lepidocampa (Diplura), Eukoenenia (Palpigrada) and Modisimus (Araneae) as troglobic in French Guiana, however their troglobic status in unknown, and all are rejected here.
VENEZUELA (45)
The most recent listing for Venezuelan troglofauna, [17], dates back to 1 and a half decades. There are 46 troglobic listed for the country, in Acari (4:9), Opiliones (4, all in Trinella, Agoristenidae), (2, both Charinus), Scorpionida (1, Taurepania trezzii, Chactidae), Diplopoda (1, Trichopolydesmidae), Collembola (1, Onychiuridae), Copepoda/Cyclopidae (1), Ostracoda (2, in Cyprididae), Bathynellaceae (1, Parabathynellidae), six Isopoda (6:6), Amphipoda (3:3), Decapoda (2, Pseudothelphusidae), Orthoptera (2, both Rhaphidiophoridae), Blattodea (1, Paranocticolla venezuelana), Coleoptera (3:3), and seven fishes (in 4 families).
From this list, we excluded the two anchialine species — Cyathura univam (Isopoda) and Metaniphargus venezuelanus (Amphipoda), as they fall outside the scope of this article.
Additionaly, new troglobic in Venezuela includes Speleopsocus chimanta Lienhard, 2010 (Psocoptera, SEE), Krenedrilus papillatus Dumnicka, 1983 (Annelida/Tubificidae) by [20], Cryptocellus armasi one recent described Ricinulei (Zootaxa, 2025), and [14] cites only five fishes, data accepted here. Thus, 45 troglobic occur in Venezuela.
COLOMBIA (42)
[19] cites 65 troglobic in Colombia, in Nematoda (1, Rhabditida), Annelida (2, Haplotaxide, Enchytraeidae one each), Tityus grottoedensis (Scorpionida), Opiliones ((7:)7), Amblypygi (2, Phrynidae and Paracharontidae), Araneae (16, in 11 families, Barychelidae, Araneidae, Anyphaenidae, Lycosidae, Tetragnathidae, Pisauridae without Brazilian troglobic), Chilopoda (3, undescribed Scolocryptops and 2 Psilliodes), Diplopoda (7), Isopoda (1, Armadillidium sp.), Coleoptera (5, two in Staphylinidae; Carabidae, Nitidulidae, Passalidae one each), Ephemeroptera (2, if confirmed, will be the first troglobis in this order worldwide), Blattoidea (1, Blaberidae), Dermaptera (1), Diptera (2, Drosophilidae and Mycetoohilidae one each), Hemiptera (3, Cydnidae, Cavernicola pilosa in Reduviidae, Strudivelia cinctipes in Veliidae), Lepidoptera (1), Trichoptera (2, in Hydrobiosidae and Hydropsychidae), Actinopteri (7, all Trichomycterus) and Avialia (Steatornis caripenis).
Steatornis caripenis assignated in work as troglobic is a obviously a huge error. Troglobic status rejecteds for both Annelida (Angarita-Sierra et al, Chapter Book, 2019), all Psilliodes (fully rejected by many sources), 13 Araneae (Angarita-Sierra et al, Chapter Book, 2019), Tityus grottoedensis (by original description, SEE), 4 Opiliones (Angarita-Sierra et al, Chapter Book, 2019), one Ephemeroptera (Angarita-Sierra et al, Chapter Book, 2019), Cavernicola pilosa (SEE) and Strudvelia cinctipes (SEE). Additionally, [14] recognizes 10 troglobic Actinopteri in country, and one Isopoda, Diploexochus cacique, was described in 2025 (SEE).
With these exceptions, Colombia has, with some consistency, 42 spp. of troglobic: Nematoda (1, Rhabditida), Opiliones (3), Amblypygi (2, Phrynidae and Paracharontidae), Araneae (3), Chilopoda (1, undescribed Scolocryptops), Diplopoda (6, in Chelodesmidae, Pyrodesmidae, Spirostreptidae, Stemmiulidae and Rhinocricidae), Isopoda (2, both in Armadillidae), Coleoptera (5, two in Staphylinidae; Carabidae, Nitidulidae, Passalidae one each), Ephemeroptera (1), Blattoidea (1, Blaberidae), Dermaptera (1), Diptera (2, Drosophilidae and Mycetoohilidae one each), Hemiptera (1), Lepidoptera (1), Trichoptera (2, in Hydrobiosidae and Hydropsychidae) and Actinopteri (10, all Trichomycterus). However, troglobic status of Nematoda, Ephemeroptera, Dermaptera, both Diptera, Lepidoptera and both Trichoptera need strong validation to be fully credited.
ECUADOR (8)
Ecuador no has unified checklist of troglobic available. Consistently, only 8 troglobic ocur in Ecuador: Marionina ecuadoriensis Righi and Hemienchytraeus mauriliae Righi (Clitellata/Enchytraeidae) by [20], Tayos ashmolei Reddell & Cokendolpher, 1984 (Schizomida, SEE), Troglotayosicus vachoni Lourenço, 1981 (Scorpiona/Troglotayosicidae), Ischnoptera peckorum Roth, 1988 (Ectobiidae, Blattaria, SEE), Pinostygus galapagoensis Cambell & Peck, 1989 (Staphylinidae, Hlavac et al., Subterranean Biology, 2006), and two Actinopteri, Astroblepus pholeter Collette 1962 and one Chaetostoma undescribed species [14].
PERU (3)
Only three spp. in Peru the following can be considered troglobic [16]: Acheroxenylla lipsae Palacios-Vargas, 2020 (Collembola, Poduromorpha, Hypogastruridae), Astroblepus riberae Cardona and Guerao, 1994 (Actinopteri, Siluriformes, Astroblepidae) and Caecopactes chullachaqui Campos-Filho, Sfenthourakis and Bichuette, 2024 (Isopoda, Scleropactidae).
BOLIVIA (8)
Bolivia no has unified checklist of troglobic available. Six troglobic are cited for Bolivia, sampled in Umajalanta cave [15]: Coleoptera (3, one described, Escolatrechus bolivianus Mateu, 2002), Acari (1, Rhagidiidae), Collembola (1, Entomobryomorpha), and Isopoda (1, Dubioniscidae). Additionally, Trichomycterus chaberti Durand 1968 and Phreatobius sanguijuela Fernandez, Saucedo, Carvajal and Schaefer 2007 [14] are troglobic in Bolivia, totalling 8 troglobics in country.
ARGENTINA (8)
Without a unified checklist of troglobic available, our survey counted eight troglobic for Argentina: Troglospinotheca (1, Collembola, Neuquen region, SEE), four Anaspidacea (SEE | SEE), one Diplopoda (Pleonaraius spelaeus, Neuquen, SEE), one Hemiptera (Notolathrus sensitivus Remes Lenicov, 1992, SEE), and one Actinopteri, Silvinichthys bortayro Fernandez and De Pinna 2005, in Trichomycteridae [14].
PARAGUAY, URUGUAY AND CHILE (2)
For Paraguay, Uruguay and Chile, only two troglobic are documented: two Anaspidacea (SEE | SEE), both from Chile.
6 BRAZILIAN NUMBERS AND TAXA
This list of cave-obligate taxa is in a continual state of flux, as new species are added through new species descriptions, some are removed by being synonymized with other species, and others are reclassified as troglophiles or stygophiles when records from surface habitats or other new evidence is obtained. Moreover, we recognize that the inclusion or exclusion of particular taxa may be contentious for some biospeleologists.
NOTES
It is noteworthy that, among the 14 troglobic species recorded from more than one state, 6 belong to Pseudoscorpiones, and several of them exhibit highly disjunct distributions that are unusual for troglobic species. This pattern raises questions about their true troglobic status or whether they may in fact represent multiple morphologically very similar species with the geographically restricted distributions typically expected for troglobites (Prado & Ferreira, Zoological Studies, 2024 — Pseudochtonius strinatii maybe three species!). For example, Spelaeochernes eleonorae has been reported n caves of Mato Grosso do Sul, Goiás, Minas Gerais and São Paulo state.
Pseudonannolene canastra (Diplopoda), a troglobitic species, was long regarded as a valid species but is currently synonymized under P. ambuatinga (Iniesta, Bouzan & Brescovit, European Journal of Taxonomy, 2023).
Some species with strong potential to be troglobic were shown, upon their formal description, not to possess this status. For example, Charinus carajas Giupponi & Miranda, 2016 and C. orientalis Giupponi & Miranda, 2016, cited by Zampulo & Prous (Fauna Cavernícola do Brasil, 2022) for Brazil, as well as Tyrannochthonius iuiu and T. aratu (Guimarães, L et al., Zootaxa, 2025), do not meet the criteria for troglobic species.
Charinus giganteus (Amblypygi, São Gabriel, Bahia, Souza & Ferreira, Zootaxa, 2025),
Pseudochthonius itakuatiara (Pseudoscorpiona, Zoological Studies, 2024),
Geogarypus gollumi and Progarypus smaugi (Pseudoscorpiona, Geogarypidae and Hesperolpiidae, respectively, Bedoya-Roqueme et al., Zootaxa, 2022),
Paracymbiomma pepita, P. otxurucu and P. una (Araneae, Cizauskas, I. et al., Zootaxa, 2024),
Pararrhopalites iataganii (Collembola, Zootaxa, 2024),
Whiteheadiana thaisae (Carabidae, Benumea et al., Zootaxa, 2023), may be troglobitic; however, the present study is unable to confirm their troglobic status.
Would Kadiweuoniscus rebellis be the species referred to as Oniscoidea 1, in Cordeiro et al. (Biota Neotropica, 2014)? No indication denying or affirming this was found in our research, thus here we consider them as distinct taxa.
TABLE

DETAILED NUMBERS
The 866 troglobic species recorded in Brazil include both described and undescribed taxa, as well as species that are either endemic to a single state or more widely distributed. Below, we provide a more detailed breakdown of each category. 350 spp. are described (208 described latter [1], or after but non cited until to date), being 337 state endemic and 13 in two or more states; and 516 remains undescribed (515 state endemic and 1 shared for two states, a Cirolanidae in Rio Grande do Norte and Ceará).
DESCRIBED IN [1]
[1] cites 150 spp., but 8 are rejected here as troglobits: Troglorhopalurus lacrau (BA, Scorpionida), Isoctenus corymbus (Ctenidae), Lygromma ybyguara (Prodidomidae), and Leonardossia hassalli (PA, Isopoda), rejected by [6]; Metagonia potiguar (RN, Araneae), non cited at Bento et al. (Biodiversity and Conservation, 2022); Ochyrocera ibitipoca (MG, Araneae), rejecyed in A. Brescovit & I. Cizauskas (Arachnology, 2018); and Endecous apterus and E. peruassuensis (rejected in Carvalho, P.H.M. et al, Zootaxa, 2023). Discocyrtus pedrosoi (BA, Opiliones), present in [1] and rejected in [6], is accepted here. Thus, [1] contributes 142 species to the new survey.
DESCRIBED AFTER [1]
A total of 208 troglobic species have been described in Brazil after [1] and are listed below by taxonomic group, with appropriate references.
PORIFERA (1) PORIFERA (1): Arinosaster patriciae (MT, Neotropical Biology and Conservation, 2021). PLATYHELMINTHES (6) PLATYHELMINTHES (8): Sluysia triapertura (RN, Invertebrates Systematics, 2018), Girardia arenicola, G. corumbataiensis, G. nobresis, G. paucipunctata, G. spelaea (SP, MS, BA, Zoologischer Anzeiger, 2021), Girardia patiensis, and G. alba (BA, Zoologischer Anzeiger, 2025). MOLLUSCA (7) BIVALVIA (1): Eupera troglobia (TO, Subterranean Biology, 2022). GASTROPODA (6): Gastrocopta sharae (GO, Zoosystematic Evolution, 2017), Habeastrum parafusum, H. omphalium (MS, Zootaxa, 2019), Spiripockia umbraticola (BA, Journal of Natural History, 2021), Idiopyrgus eowynae and I. meriadoci (BA, Zoosystematics and Evolution, 2024). ANTHROPODA/ARACNIDA (69) SCORPIONES (1): Troglorhopalurus iuiu (BA, Arthropoda, 2025). TROMBIDIFORMES (1): Leptus sidorchukae (MG, Zootaxa, 2019). PSEUDOSCORPIONA (21): Pseudochthonius ramalho, P. olegario, Spelaeochernes altamirae, S. armatus, S. bahiensis, S. dentatus, S. dubius, S. eleonorae, S. gracilipalpu, S. pedroi, S. popeye (BA, MG, SP, GO, MS, MG, BA, SE, PR, SC, Arachnology, 2022 + Revue Suisse de Zooologie, 2001), Spelaeobochicha goliath (MG, Zootaxa, 2018), S. mahnerti (MG, Zootaxa, 2020), Pseudochthonius koinopoliteia, P. diamachi, P. pali (Prado & Ferreira, Zootaxa, 2023), Pseudochthonius lubueno (BA, Zoologia, 2023), Pseudochthonius aware (BA, Zoological Studies, 2024), Pseudochthonius limettioides (MG, Zootaxa, 2024), Pseudochthonius maquinensis, and P. urubuquaqua (MG, Zootaxa, 2025). PALPIGRADES (12): Eukoenenia eywa, E. navi, E. neyriti (MG, Invertebrate Systematics, 2018), E. igrejinha (MG, Journal of Arachnology, 2019), E. ibitipoca (MG, Zootaxa, 2019), E. jequitai, E. lundi, E. magna (MG, Zootaxa, 2020), E. audax (GO, Zootaxa, 2020), Allokoenenia canhembora, A. stygia (BA, PA, European Journal of Taxonomy, 2022), and E. mocororo (MG, Zootaxa, 2022). AMBLYPYGI (5): Charinus cearensis, C. diamantinus, C. euclidesi, C. puri and C. renneri (many states, EJT, 2021). OPILIONES (4): Iandumoema cuca, I. gollum, I. stygi (MG, Invertebrate Systematics, 2020) and Spaeleoleptes gimli (BA, EJT, 2024). ARANEAE (30): Brasilomma enigmatica (MG, Zootaxa, 2012), Tonton itabirito, T. matodentro (MG, ZooKeys, 2013), Carajas paraua, Tisentnops mineiro, T. onix (PA, MG, ZooKeys, 2016), Paracymbiomma pseudocaesus (PA, Dissertation, 2017), Loxoscelis troglobia (BA, Zootaxa, 2018), Paracymbiomma caecus (PA, Zootaxa, 2018), Paracymbiomma bocaina (PA, Zootaxa, 2018), six Matta (MG, Zootaxa, 2019), Indiani gaspar (MG, Zootaxa, 2020), Ochyrocera ritxoco, O. ritxoo (PA, Zookeys, 2021), Ochyrocera dorinha, O. rosinha, O. ungoliant (MG, PA, Subterranean Biology , 2021), Speocera babau, S. pinima, S. piquira (PA, Zootaxa, 2022), Ctenus igatu (BA, Journal of Aracnology, 2022), Pinelema elinae (BA, Zootaxa, 2024), Loxosceles bodoquena (MS, EJT, 2024), Loxosceles boqueirao (BA, EJT, 2024), and Paleotoca diminas (MG, Taxonomy, 2024). ANTHROPODA/MILIPEDES (8) CHILOPODA (3): Schendylops janelao (MG, Zootaxa, 2019), Plutogeophilus jurupariquibaba (SP, Organisms Diversity & Evolution, 2023), and Cryptops didi (SP, Journal of Natural History, 2024). DIPLOPODA (5): Dobrodesmus mirabilis (BA, Zootaxa, 2016), Phaneromerium troglopterygotum (BA, Zootaxa, 2022), Strongylosomides troglobius (BA, Zootaxa, 2022), Onciurosoma troglobium (BA, Arthropoda Selecta, 2022), and Cayenniola albaserrata (BA, Zootaxa, 2024). ANTHROPODA/MALACOSTRACA (61) AMPHIPODA (3): Spelaeogammarus ginae (BA, Nauplius, 2022), S. rafaelae, S. lundi (BA/MG, Taxonomy, 2025). ISOPODA (57): Gabunillo aridicola (CE/RN, Zootaxa, 2010), Pectenoniscus lilieae, Benthana xiquinhoi (BA, Zootaxa, 2019), Amazoniscus spica, Alboscia jotajota, Metaprosekia igatuensis (PA, SP, BA, European Journal of Taxonomy, 2020), Xangoniscus aganju, X. lundi, X. dagua, X. santinhoi, X. ceci (BA, MG, Zootaxa, 2020), Pectenoniscus montalvaniensis, P. juveniliensis, P. iuiuensis, P. carinhanhensis, Pectenoniscus santanensis, P. morrensis (BA, MG, Nauplius, 2020), Chaimowiczia tatus, C. uai (BA, MG, Subterranean Biology, 2021), Pectenoniscus fervens, P. pankaru (PI, BA, ZooKeys, 2022), Spelunconiscus septemlacuum (MG, Nauplius, 2022), Xangoniscus lapaensis, X. loboi (BA, Subterranean Biology, 2022), Chaimowiczia obybytyra (BA, Subterranean Biology, 2022), Iansaoniscus leilae, I. paulae (BA, Subterranean Biology, 2022), Cylindroniscus platoi (MG, Zootaxa, 2022), Benthana alba, Benthanoides amazonicus, B. tarzan (PA, Zootaxa, 2023), Pectenoniscus monsviridis, P. revelatus, P. archaeos, P. sepultus, P. caesarius (BA, Studies on Neotropical Fauna and Environment, 2023), Caraiboscia jabutiensis, Gabunillo enfurnado, Venezillo moreirai, V. limai (many states, Zoosystema, 2023), Trichorhina baiana (BA, Biota Neotropica, 2023), Kadiweuoniscus rebellis (MS, ZooKeys, 2024), Circoniscus mendesi, C. xikrin (PA, EJT, 2024), Ctenorillo iuiuensis (BA, Zoosystema, 2024), Xangoniscus paiabare, X. tymaopeba, X. ykanhema, X. puku (BA, Tropical Zoology, 2024), Xangoniscus antiquus, X. chaimowiczi, X. jonasi (BA, Subterranean Biology, 2024), Oiticicarcinia epikarstica, Itararecarcinia yapyra, Poicarcinia jandairensis (RN, Tropical Zoology, 2025), Circoniscus paradisus (PA, Nauplius, 2025), and Brasileirinho sergipanus (SE, Zootaxa, 2025). DECAPODA (1): Aegla charon (SP, Nauplius, 2017). ANTHROPODA/COLLEMBOLA (25) COLLEMBOLA (25): Pararrhopalites sideroicus (MG, Florida Entomologist, 2014), Arrhopalites glabrofasciatus, Pseudosinella guanhaensis (MG, Zootaxa, 2018), Pararrhopalites ubiquum (MG, Neotrop. Entomol., 2018), 10 in Trogolaphysa, 9 in Pseudosinella (PA, MG, SP, Insects, 2020, and PRE-PRINT, 2021), Troglobentosminthurus luridus (BA, Insects, 2022), and Cyphoderus caatinguensis (RN, Zootaxa, 2024). ANTHROPODA/INSECTA (22) ORTHOPTERA (5): Endecous troglobia (MG, Zootaxa, 2020), Erebonyx catacumbae (BA, Zootaxa, 2021), Endecous infernalis (BA, Zootaxa, 2023), Endecous vitreus (MS, Zootaxa, 2023), and Endecous spelaeus (BA/Feira da Mata, Zootaxa, 2025). COLEOPTERA (11): Ardistomis ferreirai (PA, Zootaxa, 2018), Oxarthrius aurora, O. inexpectatus (TO, BA, Zootaxa, 2018), Metopioxys carajas (PA, Zootaxa, 2019), Coarazuphium lundi (MG, Zootaxa, 2020), C. auleri (MT, Studies on Neotropical Fauna and Environment, 2021), C. bambui (BA, Zootaxa, 2022), C. xingu, C. xikrin, C. kayapo (PA, Subterranean Biology, 2022), and Perigona spelunca (MG, EJT, 2022). DERMAPTERA (1): Mesodiplatys falcifer (BA, ZooKeys, 2018). DIPTERA (1): Deanemyia maruaga (AM, Mem. Inst. Oswaldo Cruz, 2011). HEMIPTERA (5): Spelaeometra gruta, Cephalometra pallida (BA, Tijdschrift voor Entomologie, 2018), Ferricixius michaeli, F. goliathi (MG, Zootaxa, 2023), Spelaeometra hypogea (BA, Animals, 2023). CRANIATA (1) ACTINOPTERI (1): Phreatobius sanguijuela (RO/Bolivia, Fauna Cavernicola do Brasil, 2022).
UNDESCRIBED
518 troglobic species remains undescribed, species identifiable as troglobic but not yet formally described, but with certainty or very high signs of troglomorphisms, distributed along following state/references below.
BY REFERENCE AND STATE
Codes in bold, as [MG/4], will be used later to cite these works throughout the group discussion.
GOIÁS
[GO/1] — 12 caves in São Domingos and Posse municipalities — 10 undescribed — Bichuette, ME et al (Subterranean Biology, 2019).
Pseudoscorpiona/Spaleochernes (1), Diplopoda/Pseudonanolene (2), Polydesmida (1), Isopoda/Styloniscidae (1), Isopoda/Dubioniscidae (1), Collembola/Cyphoderus (1), Collembola/Trogolaphysa (2), and Coleoptera/Pselaphinae (1).
[BR/2] — Posse and São Domingos municipalities — 3 undescribed — Bichuette & Gallão (Neotropical Ichthyology, 2021).
Actinopteri/Ituglanis (2), Actinopteri/Phenocorhamdia (1).
MATO GROSSO DO SUL
[MS/1] — 84 caves Bodoquena region and Pantanal region — 31 undescribed — Cordeiro et al. (Biota Neotropica, 2014).
Giradia (1), Oligochaeta (2), Gastropoda/Spiripockia (1), Gastropoda/Pomatiopsidae (1), Onychophora/Peripatopsidae (1), Acari/Hydrozetes (1), Opiliones/Eusarcus (6), Araneae/Ctenidae (1), Paradoxosomatidae (2), Diplopoda/Kantanodesmus (1), Diplopoda/Cryptodesmus (1), Amphipoda/Megagidiella (1), Bathynellacea (1), Isopoda/Oniscidae (1), Collembola/Trogolaphysa (1), Collembola/Entomobryiomorpha (2), Collembola/Cyphoderidae (1), Collembola/Paronellidae (1), Coleoptera/Pachyteles (1), Hemiptera/Dipsocariidae (1), Actinopterii/Rhamdia (1), Actinopterii/Ancistrus (1), and Actinopterii/Trichomycterus (1).
[BR/1] — Abismo cave in Jardim municipality — 1 undescribed — Zepon, T et al. (Paíes Avulsos de Zoologia, 2025).
Coleoptera/Hydroscaphidae (1).
PARÁ
[PA/1] — Paraíso cave, Aveiro — 9 undescribed — Souza-Silva, M et al. (ANAIS do 36º Congresso Brasileiro de Espeleologia, 2022).
11 original undescribed, 10 remains undescribed: Glomeridesmus (1), Pyrgodesmidae (2), Cryptodesmidae (1), Fuhrmannodesmidae (1), Oniscodesmidae (1), Polydesmida (1), Scleropactidae (2), and Escadabiidae (1).
Of the 11 species in the original study, one has already been formally described: Circoniscus sp. 2 as Circoniscus paradisus (Galo, JB et al., Nauplius, 2025).
[PA/2] — Carajas region of SE Pará — 122 undescribed — a compilated list of many studies of 473 caves in Canaã dos Carajás (85 caves), Parauapebas (296 caves) and Curionópolis (90 caves) in Trevelin et al. (Ecological Indicators, 2019).
Isotomiella numulifer Deharveng & Oliveira, 1990 (Collembola, Isotomidae), which had been cited in the study for Curionópolis, is here excluded, as there is no evidence that it is a troglobic species (occur in forests, SEE). The study cites Charinus carajas Giupponi & Miranda, 2016 and C. orientalis Giupponi & Miranda, 2016 as troglobic, but this status is rejected for both by [6]. Trogolaphysa dandarae Brito & Zeppelini and T. gisbertae Brito & Zeppelini (SEE) may have been described from populations of Paronellidae sp. 4, although there is no concrete reference indicating whether the populations from Canaã dos Carajás were later described under a different name. Although Amazoniscus spica Campos-Filho, Aguiar & Taiti (SEE), Circoniscus mendesi López-Orozco, Campos-Filho & Bichuette, and C. xikrin López-Orozco, Campos-Filho & Carpio-Díaz (SEE), all Scleropactidae, were described from Pará after that study, there is no evidence that they correspond to Crinocheta sp. 1 or Amazoniscus sp. 1; therefore, all of them are treated here as distinct species. Metopioxys carajas Asenjo, described after the study (SEE), does not, based on the available evidence, correspond to any of the 13 undescribed Staphylinidae species listed in the work (neither the suspect Staphylinidae sp. 8). Coarazuphium kayapo Pellegrini, Ferreira & Vieira and C. xikrin Pellegrini, Ferreira & Vieira, described after the study (SEE), show no evidence of corresponding to any of the 9 undescribed Carabidae species recorded in the survey. Although Paracymbiomma bocaina Rodrigues, Cizauskas & Rheims, 2018 and P. caecus Rodrigues, Cizauskas & Rheims, 2018 could represent Lygromma sp. 2 due to the occurrence of all three in two caves of Canaã dos Carajás (SEE), the evidence goes no further, and therefore we treat the three as distinct species. The wide distribution of Speocera piquira Brescovit, Zampaulo, Cizauskas & Pedroso, 2022 and S. babau Brescovit, Zampaulo, Cizauskas & Pedroso, 2022 provides evidence that neither of them corresponds to any of the four undescribed Ochyroceratidae cited in the study. S. pinima Brescovit, Zampaulo, Cizauskas & Pedroso, 2022 may correspond to Ochyroceratidae sp. or Ochyroceratidae sp. 5, but stronger evidence is lacking (SEE). Very little can be said about Ochyrocera ritxoco Brescovit, Zampaulo & Cizauskas, 2021, O. ritxoo Brescovit, Zampaulo & Cizauskas, 2021 (SEE), O. ungoliath Brescovit, Cizauskas & Mota, 2018 (SEE), and Araneae sp. G . All 11 species are treated here as distinct.
Thus, of the 133 species cited in the study (noting that not all troglobic species from the three municipalities were included in the survey), 3 are excluded, 8 have already been formally described, and 122 are here considered as undescribed troglobic species.
Undescribed: Oligochaeta (3), Tricladida (1). Geoplanidae (1), Systrophiidae (3), Arachnida (27), Chilopoda/Cryptops (1), Diplopoda (18), Isopoda (16), Collembola (16), Coleoptera (38), Formicidae/Rogeria (1), Hemiptera/Dipsocoridae (2).
TOCANTINS
[TO/1] — 4 caves in Dianópolis — 6 undescribed — Ferreira, RL et al. (CECAV, 2016).
Araneae/Nesticidae (1), Araneae/Symphytogmatidae/Anapistula (1) Polydesmida (1), Styloniscidae (1), Coleoptera/Scydmaeninae (1), Formicidae/Amblyopone (1).
RIO GRANDE DO NORTE
[RN/1] — 40 caves of W Rio Grande do Norte state in Felipe Guerra, Baraunas, Apodi and Governador Dix Sep-Rosado municipalities — 50 undescribed , 49 exclusive and one shared with Ceará state — Bento D et al. (Biodiversity and Conservation, 2022 | EXCEL LIST).
51 original undescribed, 50 remains undescribed: Hausera (1), Oligochaeta (2), Araneae (2), Ochyroceratidae (2), Oonopidae (1), Pholcidae (1), Prodidomidae (1), Chthoniidae (1), Opiliones/Gonyleptidae (1), Chilopoda/Lithobiomorpha (1), Chilopoda/Dinocryptops (4), Chilopoda/Geophilomorpha (1), Polydesmida (2), Cryptodesmidae (1), Ostracoda (1), Copepoda (2), Amphipoda/Potiberaba (4), Isopoda/Cirolanidae (6), Isopoda/Trichorhina (1), Isopoda/Styloniscidae (1), Collembola/Arrhopalites (1), Collembola/Cyphoderus (6), Collembola/Pseudosinella (2), Diplura (1), Japygidae (2), Coleoptera/Carabidae (1) and Coleoptera/Staphylinidae (1).
Originally, there were 51 undescribed species, but one, Calabozoida sp., was described as Oiticicarcinia epkaristica (Cardoso, GM et al., Tropical Zoology, 2025).
BAHIA
[BA/1] — 4 caves in Água Clara cave system, Carinhanha municipality — 18 undescribed — Souza-Silva, M et al. (Biodiversity and Conservation, 2021).
Gastropoda/Pulmonata (1), Trombidiformes/Rhagidiidae (1), Araneae/Caponiidae (1), Araneae/Ochyroceceratidae (1), Eukoenenia (1), Diplopoda/Trichopolydesmidae (1), Diplopoda/Pyrgodesmidae (1), Chilopoda/Geophilomorpha (1), Isopoda/Styloniscidae (3), Isopoda/Trichorhina (1), Collembola/Sminthuridae (1), Collembola/Entomobryomorpha (2), Collembola/Symphypleona (1), Insecta/Blattodea(1), Insecta/Nylanderia (1).
[BA/2] — 18 caves from Iuiu and Malhada — 32 undescribed — Cardoso, RC et al. (Biodiversity and Conservation, 2021).
Gastropoda/Scolodontidae (1), Gastropoda (3), Araneae/Ochyroceratidae (1), Pseudoscorpiona/Spelaeobochica (1), Pseudoscorpiona/Chthoniidae (1), Eukoenenia(2), Opiliona/Acantholibinia (1), Opiliona/Zalmoxidae (1), Diplopoda/Pyrgodesmidae (3), Diplopoda (1), Diplopoda/Chelodesmidae (1), Diplopoda/Paradoxosomatidae (1), Diplopoda/Pseudonannolene (1), Chilopoda/Geophilomorpha (1), Chilopoda/Lithobiomorpha (1), Chilopoda/Cryptops (1), Isopoda/Styloniscidae (5), Isopoda/Pectenoniscus (1), Collembola/Arrhopalites (1), Diplura/Projapygidae (1), Insecta/Nicoletiidae (1), Insecta/Blattodea (1), and Coleoptera/Clivinina (1).
[BA/3] — five caves from Igatu region in Andaraí and Lençóis municipalities — 29 undescribed — Gallão, JE et al. (Diversity, 2023).
Gastropoda/Happia (1), Mesostigmata/ Pachylaepidae (1), Mesostigmata/Dithinozeronidae (1), Sarcoptiformes/Oeserchestidae (1), Araneae/Prodidominae (1), Araneae/Ochyrocera (1), Araneae/Metagonia (1), Araneae/Telemidae (1), Pseudoscorpiona/Spelaeochernes (1), Pseudoscorpiona/Pseudochthonius (1), Pseudoscorpiona/Syarinidae (1), Eukoenenia (1), Opiliona/Tricommatidae (1), Diplopoda/Chelodesmidae (1), Diplopoda/Crypturodesmus (1), Chilopoda/Sphendononema (1), Chilopoda/Cryptops (1), Isopoda/Philosciidae (1), Isopoda/Platyartridae (2), Collembola/Verhoeffiella (1), Collembola/Heteromurini (1), Collembola/Troglopedetes (1), Diplura/Projapygidae (1), Insecta/Nicoletiidae (1), Insecta/Blattelidae (1), Coleoptera/Scydmaenidae (1), Coleoptera/Pselaphinae (1), and Actinopteri/Copionodon (1).
.
[BA/4] — Toca do Gonçalo cave in Campo Formoso — 14 undescribed — Sousa Silva & Ferreira (Subterranean Biology, 2016).
15 original undescribed, 14 remains undescribed: Gastropoda/Rotadiscus (1), Araneae/Lygromma (1), Pseudoscorpiona/Chthoniidae (1), Diplopoda/Polyxenida (1), Diplopoda/Oniscodesmidae (1), Chilopoda/Geophilomorpha (1), Isopoda/Scleropactidae (1), Isopoda/Trichorhina (1), Collembola/Arrhopalites (1), Insecta/Ortheziidae (1), Insecta/Nicoletiidae (1), Coleoptera/Clivinina (1), Isopoda/Dyticidae (1), and Actinopteri/Rhamdiopsis (1).
Originally, there were 15 undescribed species, but one was described latter: Erebonix catacumbae (former Phalangospsidae sp. n., Melo & Ferreira, Zootaxa, 2021).
[BA/5] — Carinhanha region — 10 undescribed — Ferreira & Souza-Silva (Diversity, 2023).
Araneae/Palpimanidae (1), Araneae/Ochiroceratidae (1), Araneae/Matta (1), Diplopoda/Siphonophoridae (1), Diplopoda/Trichopolydesmidae (1), Diplura/Projapygidae (1), Hemiptera/Delphacidae (1), Coleoptera/Clivinina (1), Coleoptera/Trechinae (1), and Hemiptera/Spelaeometra (1).
[BA/6] — Pedro Cassiano cave in Carinhanha municipality — 13 undescribed — Souza-Silva, M et al. (Acta Oecologica, 2025).
Araneae/Ochyroceratidae (1), Araneae/Oonopidae (1), Eukoenenia (1), Polydesmida (1), Isopoda/Pectenoniscus (1), Isopoda/Xangoniscus (1), Collembola/Symphypleona (1), Collembola/Neelipleona (1), Collembola/Trogolaphysa (2), Insecta/Blattodea (1), and Coleoptera/Clivinina (2)
[BA/7] — Padre Cave in Santana municipality — 20 undescribed — Pereira Junta, VG et al. (Community, 2025).
Nemertea (1), Oligochaeta (1), Gastropoda/Spiripockia (1), Araneae/Ochyroceratidae (1), Opiliona/Escadabiidae (1), Opiliona/Eusacus (1), Eukoenenia (2), Symphyla (1), Isopoda/Platyartridae (1), Isopoda/Styloniscidae (1), Isopoda/Xangoniscus (1), Collembola/Paronellidae (1), Collembola/Poduromorpha (1), Collembola/Arrhopalitidae (1), Insecta/Blattodea (1), Insecta/Coleoptera (1), Insecta/Orthoptera (1), and Actinopteri/Pimelodella (1).
[BR/2] — Coribe, Iuiu, Feira da Mata, Mirangaba and Umburanas — 6 undescribed — Bichuette & Gallão (Neotropical Ichthyology, 2021).
Trichomycterus (2), Rhamdiopsis (4).
ESPÍRITO SANTO
[ES/1] — 15 caves in Santa Teresa, Ecoporanga, Pedro Calvário, Jaciguá, Vargem Alta and Conceição do Castelo municipalities — 10 undescribed — Souza Silva & Ferreira (Subterranean Biology, 2015).
Araneae/Ochyroceratidae (1), Escadabiidae (2), Pseudonannolene (1), Cyrtodesmidae (1), Trichopolydesmidae (1), Isopoda/Trachelypodidae (1), Isopoda/Trichorhina (1), and Zygentoma (2).
MINAS GERAIS
[MG/1] — 15 limestone caves located in the municipalities of Cordisburgo (13 caves) and Curvelo (2 caves) — 35 undescribed — Souza, MVR et al. (International Journal of Speleology, 2021 | SUPPLEMENTARY MATERIAL).
Nemertea (1), Annelida/Hirudinea (1), Mesostigmata (1), Araneae/Ochyroceratidae (1), Araneae/Oonopidae (1), Araneae/Prodidomidae (1), Araneae/Oonopidae (1), Pseudoscorpiona/Chthoniidae (2), Pseudoscorpiona/Pseudochthonius (1), Opiliones/Spinopilar (1), Opiliones/Spaeleoleptes (1), Eukoenenia (1), Diplopoda/Oniscodesmidae (1), Diplopoda/Hypogexenidae (1), Diplopoda/Pyrgodesmidae (1), Diplopoda/Trichopolydesmida (1), Diplopoda/Polydesmida (2), Pauropoda (1), Isopoda/Styloniscidae (2), Isopoda/Pectenoniscus (3), Isopoda/Trichorhina (2), Collembola/Arrhopalites (2), Collembola/Cyphoderidae (1), Diplura/Japygidae (1), Insecta/Zygentoma (1), Coleoptera/Trechinae (1), Coleoptera/Pselaphinae (1), and Coleoptera/Coarazuphuim (1).
[MG/2] — 20 caves from Ibitipoca Estadual Park in Lima Duarte and Santa Rita do Ibitipoca — 5 undescribed — Souza Silva, Iniesta and Ferreira (Subterranean Biology, 2020).
Collembola/Hypogastruridae (1), Diplura/Projapygidae (2), Insecta/Blattodea (1), and Coleoptera/Pselaphinae (1).
[MG/3] — 9 caves in Belo Horizonte, Nova Lima, Ibirité e Brumadinho — 9 undescribed — Zampaulo (33rd CBE, 2015).
Araneae (Tsentinops-1, Lygromma-1), Opiliones/Spinopilar (1), Pseudoscorpiones/Pseudochthonius (1), Diplopoda/Pyrgodesmidae (1), Collembola (3, Pararrhopalites-1, Pseudosinella-1, Trogolaphysa-1), and Coleoptera/Pselaphinae (1).
[MG/4] — 6 caves in Sumidouro State Park, Lagoa Santa municipality — 2 undescribed — Iniesta, LFM et al. (Revista Brasileira de Espeleologia, 2012).
Trombidiformes/Labidostomatidae (1) and Isopoda/Trichorhina (1).
[MG/5] — 14 caves from Peruaçu Caves National Park, in Itacarambi and Januaria municipalities — 2 undescribed — Monte & Bichuette (Biota Neotropica, 2020).
Diplopoda/Oniscodesmidae (1) and Collembola (1).
[MG/6] — Éden cave in Pains municipality — 14 undescribed — Ratton Sousa, P et al. (Revista Brasileira de Espeleologia, 2017).
Opiliones/Paratricommatus (1), Pseudoscorpiones/Chthoniidae (1), Diplopoda/Sphaerodesmidae (1), Symphyla (3), Isopoda/Styloniscidae (2), Copepoda/Harpacticoida (1), Collembola/Arrhopalites (1), Insecta/Blattellidae (1), and Coleoptera/Carabidae (3).
[MG/7] — 7 caves in Arinos municipality — 7 undescribed — Henrique Simões, M. et al. (Revista Brasileira de Espeleologia, 2012).
Turbellaria (1), Annelida/Hirudinea(1), Diplopoda/Oniscodesmidae (1), Diplopoda/Polyxenidae (1), Collembola (1), Isopoda/Trichorhina (1), and Isopoda/Styloniscidae (1).
[MG/8] — 7 caves in Presidente Olegário municipality — 3 undescribed — Zepon (Master’s Thesis/UFSCar, 2015).
4 original undescribed, 3 remains undescribed: Polydesmida (1), Coleoptera/Pselaphinae (1), and Coleoptera/Carabidae (1).
Originally, there were 4 undescribed species, but one, Chthoniidae sp. 4, was described as Pseudochthonius olegario (von Schimonsky, DM et al., Arachnology, 2022).
[MG/9] — Matosinhos municipality — 4 undescribed — Cunha Santos, NM (Master’s Thesis/UFRN, 2020).
Collembola/Trogolaphysa (4).
[MG/10] — Lapa Nova cave, Vazante municipality — 5 undescribed — Pellegrini & Ferreira (Subterranean Biology, 2016).
Araneae/Oonopidae (1), Pseudoscorpiones/Chthoniidae (1), Collembola/Arrhopalites (1), Collembola/Acherontides (1), and Isopoda/Styloniscidae (1).
[BR/2] — São Rique de Minas and Cordisburgo — 2 undescribed — Bichuette & Gallão (Neotropical Ichthyology, 2021).
Ituglanis (1), Rhamdiopsis (1).
SÃO PAULO
[SP/1] — 33 caves in Alto Ribeira karst in Iporanga, Apium and Guapira municipalities — 6 undescribed — Bichuette & Trajano (Subterranean Biology, 2018).
Potamolithus (6).
[SP/2] — three caves in Areias Cave System, Iporanga — 13 undescribed — Souza Silva & Ferreira (Subterranean Biology, 2016).
Girardia (1) Nemertea (1), Araneae/Hahniidae (1), Eukoenenia (1), Cryptodesmidae/Cryptodesmus (1), Oniscodesmidae/Crypturodesmus (1), Pyrgodesmidae/Peridontosmella (1), Isopoda/Trichorhina (2), Collembola/Falsomia (1), Collembola/Cyphoderidae (1), Collembola/Paronellidae (1), and Coleoptera/Carabidae (1).
[SP/3] — Riacho Subterrâneo cave in Itu municipality — 12 undescribed — Bichuette, ME et al. (Neotropical Biology and Conservation, 2017).
Chthoniidae (1), Chelodesmidae (5), Polydesmidae (2), Geophilidae (1), Symphyla (1), Copepoda (1), Collembola/Entomobryiomorpha (1).
[SP/4] — two caves in Bulhas d'água regio, Iporanga municipality — 1 undescribed — Zepon, T et al. (Espeleo-Tema, 2019).
Paradoxosomatidae (1).
[BR/2] — Juquiá municipality — 1 undescribed — Bichuette & Gallão (Neotropical Ichthyology, 2021).
Pimelodella (1).
PARANÁ
[PR/1] — 12 limestone caves in Itaiacoca formation in NE Paraná state in Dr. Ulysses, Castro, Cerro Azul and Sengès municipalities — 13 undescribed — Mata Melo, L et al. (37th CBE, 2023).
Gastropoda (1), Araneae/Prodidomidae (1), Opiliones (2), Palpigrada (3), Chthoniidae (1), Crypturodesmus (1) Pyrgodesmidae (3) and Isopoda/Trichorhina (1).
UNVAILABLE ARTICLES
48 caves Bambuí system in Minas Gerais state — unknown numebr of undescribed — Rabelo, LM et al. ( Biodiversity and Conservation, 2018).
Unknown list.
BY GROUP
The highest numbers by undescribed troglobic in Brazil includes Insecta (88), Diplopoda (81), Collembola (67), Isopoda (66), Araneae (46), and Opiliones (27).
PLATYHELMINTHES (6)
PA (2), SP (1), MS (1), RN (1), MG (1).
NEMERTEA (3)
BA (1), SP (1), MG (1).
ANNELIDA (10)
PA (3), MS (2), MG (2), RN (2), BA (1).
MOLLUSCA (20)
BA (8), SP (6), PA (3), MS (2), PR (1).
ONYCHOPHORA (1)
MS (1).
ARTHROPODA
ARACHNIDA
Acari (9) ‣ BA (4), MG (2), PA (2), MS (1).
Araneae (46) ‣ BA (14), PA (12), MG (7), RN (7), ES (1), SP (1), MS (1), TO (2), PR (1).
Pseudoscorpionida (21) ‣ BA (6), MG (6), PA (5), RN (1), SP (1), GO (1), PR (1).
Palpigrada (12) ‣ BA (7), PR (3), MG (1), SP (1).
Schizomida (1) ‣ PA (1).
Opiliones (27) ‣ PA (8), MS (6), MG (4), BA (5), ES (2), RN (1).
MYRIAPODA
Pauropoda (1) ‣ MG (1).
Symphyla (5) ‣ MG (3), BA (1), SP (1).
Diplopoda (81) ‣ PA (18), BA (16), MG (12), SP (11), PA (6), MS (4), PR (4), ES (3), RN (3), GO (3), TO (1).
Chilopoda (15) ‣ BA (7), RN (6), SP (1), PA (1).
OLIGOSTRACA
Ostracoda (1) ‣ RN (1).
MALACOSTRACA
Amphipoda (5) ‣ RN (4), MS (1).
Bathynellacea (1) ‣ MS (1).
Isopoda (66) ‣ BA (21), MG (13), PA (12), RN (8, 1 non-endemic), SP (2), ES (2), GO (2), PA (2), MS (1), TO (1), PR (1), CE (1, non-endemic).
COPEPODA
Copepoda (5) ‣ RN (2), SP (1), BA (1), MG (1).
HEXAPODA
Collembola (67) ‣ MG (16), BA (16), PA (16), RN (9), MS (5), SP (4), GO (3).
Diplura (9) ‣ MG (3), RN (3), BA (3).
Insecta (88)
Zygentoma ‣ BA (3), ES (2), MG (1).
Coleoptera ‣ PA (38), MG (10), BA (9), RN (2), MS (2), SP (1), GO (1), TO (1).
Blattodea ‣ BA (5), MG (2).
Hemiptera ‣ MS (1), BA (3), PA (1).
Orthoptera ‣ BA (1).
Hymenoptera ‣ BA (1), TO (1), PA (1).
ACTINOPTERI (18)
BA (9), MS (3), GO (3), MG (2), SP (1).
BRAZILIAN TROGLOBIC BY GROUP
Highest diverse group in troglobic species in Brazil are: Isopoda (136), Collembola (108), Diplopoda (94), Coleoptera (88), Araneae (81), Pseudoscorpiona (49), Opiliones (43), Actinopteri (40), Palpigradi (30), Gastropoda (28), Amphipoda (23), Chilopoda (23), Platyhelminthes (17), Hemiptera (13), Amblypygi (11) and Diplura (10).
BRAZILIAN TROGLOBIC BY STATE
Only 14 troglobic in Brazil are non states-endemic: Brazil/Bolivia (1, Phreatobius sanguijuela), São Paulo/Paraná (3), Ceará/Rio Grande do Norte (3), Pará/Amapá (1, Phreatobius cisternarum), Minas Gerais/São Paulo (1, Spelaeochernes gracilipalpus), Bahia/Sergipe (1, Spelaeochernes popeye), Mato Grosso do Sul/Mato Grosso (1, Potiicoara brasiliensis), Minas Gerais/São Paulo/Paraná (1, Pseudochthonius strinatii), Minas Gerais/São Paulo/Mato Grosso do Sul (1, Spelaeochernes dubius) and Minas Gerais/São Paulo/Goiás/Mato Grosso do Sul (1, Spelaeochernes eleonorae). Six of these species are Pseudoscorpiones.
If you count described and undescribed species state endemic, the numbers are: Bahia (250), Minas Gerais (189), Pará (170), São Paulo (66), Rio Grande do Norte (58), Mato Grosso do Sul (43), Goiás (27), Paraná (17), Espírito Santo (11), Tocantins (9), Mato Grosso (4), Ceará (2), Santa Catarina (2), Rio de Janeiro (2), Piauí (1), Amazonas (1), Rondonia (1) and Sergipe (1). Amapá no has endemic troglobic.
If we count all the species (including non state endemic), we have, the numbers are Bahia (251), Minas Gerais (193), Pará (171), São Paulo (73), Rio Grande do Norte (61), Mato Grosso do Sul (46), Goiás (28), Paraná (21), Espírito Santo (11), Tocantins (9), Ceará (5), Mato Grosso (5), Santa Catarina (2), Rondonia (2), Rio de Janeiro (2), Amazonas (1), Piauí (1), Sergipe (2) and Amapá (1).
Undescribed for state: Bahia (142), Pará (131), Minas Gerais (88), Rio Grande do Norte (50, one non-endemic), São Paulo (33), Mato Grosso do Sul (32), Espírito Santo (10), Goiás (13), Tocantins (6) and Ceará (1, non-endemic). 518 spp., all but one (Cirolanidae) state endemic.
TROGLOBIC BY MUNICIPALITY
Most distributions followed the original descriptions of the species, or local inventories. Another important source consulted was Pires et al. (CBE, 2015) and [1]. 162 municipalities has troglobic in Brazil based on our survey. The species that was identified in the most municipalities was Trogolaphysa bellinii (Collembola/Paronellidae), having been identified in 10 municipalities in Minas Gerais state.
AM [1] ‣ Presidente Figueredo (1).
RO [2] ‣ Porto Velho (1) and São Francisco do Guaporé (1).
AP [2] ‣ Amapá (1) and Macapá (1).
PA [13] ‣ Parauapebas (87), Curionópolis (51), Canaã dos Carajás (39), Aveiro (10), São Felix do Xingu (3), Altamira (2), Medicilândia (3), Ananindeua (1), Belém (1), Benfica (1), Brasil Novo (1), Eldorado dos Carajás (1), and Salvaterra (1).
PI [1] ‣ Coronel José Dias (1).
CE [4] ‣ Quixerê (2), Santa Quitéria (1), Aiuaba (1), and Ubajara (1).
RN [5] ‣ Felipe Guerra (37), Dix-Sept Rosado (13), Baraúna (8), Apodi (4), and Jandaira (1).
SE [3] ‣ Japaratuba (1), Lagarto (1), and Laranjeiras (1).
BA [29] ‣ Carinhanha (59), Andaraí (38), Iuiu (34), Santana (28), Campo Formoso (27), Malhada (20), Coribe (11), Feira da Mata (7), Iraquara (7), Itaetê (6), Serra do Ramalho (6), São Desidério (4), Morro do Chapéu (3), Palmeiras (3), Paripiranga (3), Varzea Nova (3), Lençóis (2), Miranguara (2), Santa Maria da Vitória (2), Bom Jesus da Lapa (1), Canálopis (1), Curaçá (1), Ituaçu (1), Maria da Vitória (1), Mucugê (1), Nova Redenção (1), Ourolândia (1), Pau Brasil (1), Umburana (1).
TO [4] ‣ Dianópolis (6), Aurora de Tocantins (1), Lagoa da Confusão (1), and Natividade (1).
MT [4] ‣ Nobres (2), Curvelândia (1), Rosario do Oeste (1), and Diamantino (1).
GO [8] ‣ São Domingos (18), Posse (5), Mambaí (3), Buritinópolis (1), Corumbá de Goiás (1), Guarani de Goiás (1), Nova Roma (1), Pedro Bernardo (1).
MG [54] ‣ Cordisburgo (42), Pains (17), Itacarambi (16), Matozinhos (11), Rio Acima (11), Belo Horizonte (10), Itabirito (9), Mariana (8), Arinos (7), Pedro Leopoldo (7), Barão de Cocais (6), Brumadinho (6), Morro do Pilar (6), Nova Lima (6), Sete Lagoas (6), Vazante (6), Conceição do Mato Dentro (5), Lima Duarte (5), Montes Claros (5), Presidente Olegário (5), Caete (4), Luislandia (4), Santa Bárbara (4), Curvelo (3), Lagoa Santa (3), Prudente de Morais (3), Ibiracatu (2), Itambé do Mato Dentro (2), Monjolos (2), Prados (2), São Gonçalo do Rio Abaixo (2), São Roque de Minas (2), Arcos (1), Capim Branco (1), Carai (1), Catas Altas (1), Dores de Guanhaes (1), Jaiba (1), Jequitaí (1), Juvenilia (1), Moeda (1), Montalvania (1), Ouro Preto (1), Piumhi (1), Presidente Juscelino (1), Rio Pardo de Minas (1), Santa Maria de Itabira (1), São João da Lagoa (1), São João da Lapa (1), São João da Ponte (1), São Sebastião do Rio Preto (1), Unaí (1), and Vespasiano (1).
ES [5] ‣ Vargem Alta (5), Ecoporanga (2), Santa Teresa (2), Conceição do Castelo (1), and Pedro Calvário (1).
RJ [2] ‣ Cantagalo (1) and Cambuci (1).
SP [12] ‣ Iporanga (52), Itu (12), Eldorado (3), Itirapina (2), Guapiara (2), Altinopólis (1), Analandia (1), Apiai (1), Ipeuna (1), Juquiá (1), Ribeirão Grande (1), and Ribeira (1).
MS [5] ‣ Bonito (24), Bodoquena (23), Jardim (6), Porto Murtinho (3), and Corumbá (3).
PR [8] ‣ Dr. Ulysses (9), Castro (7), Cerro Azul (4), Sengés (4), Rio Branco do Sul (3), Adrianópolis (2), Almirante Tamandaré (1), and Ponta Grossa (1).
SC [1] ‣ Botuverá (2).
RANKING BY MUNICIPALITY
Largest diversities by municipalities (all species): Parauapebas (87), Carinhanha (59), Iporanga (52), Curionópolis (51), Cordisburgo (42), Canaã dos Carajás (39), Andaraí (38), Felipe Guerra (37), Iuiu (34), Santana (28), Campo Formoso (27), Bonito (24), Bodoquena (23), Malhada (20), São Domingos (18), Pains (17), Itacarambi (16), Governador Dix-Sept Rosado (13), Itu (12) — Coribe (11), Matozinhos (11), Rio Acima (11) — Aveiro (10), Belo Horizonte (10) — Dr. Ulysses (9), Itabirito (9) — Baraúna (8), Mariana (8) — Arinos (7), Castro (7), Feira da Mata (7), Iraquara (7), Pedro Leopoldo (7), Serra do Ramalho (7) — Barão de Cocais (6), Brumadinho (6), Dianópolis (6), Itaetê (6), Jardim (6), Montes Claros (6), Morro do Pilar (6), Nova Lima (6), Sete Lagoas (6), Vazante (6) — Conceição do Mato Dentro (5), Lima Duarte (5), Posse (5), Presidente Olegário (5), Vargem Alta (5) — Apodi (4), Caete (4), Cerro Azul (4), Luislandia (4), Santa Bárbara (4), São Desidério (4), Sengés (4) — Caete (3), Corumbá (3), Curvelo (3), Eldorado (3), Lagoa Santa (3), Mambaí (3), Morro do Chapéu (3), Palmeiras (3), Paripiranga (3), Porto Murtinho (3), Prudente de Morais (3), Rio Branco do Sul (3), São Felix do Xingu (3), Varzea Nova (3) — Adrianópolis (2), Altamira (2), Botuvera (2), Ecoporanga (2), Guapiara (2), Ibiracatu (2), Itambé do Mato Dentro (2), Itirapina (2), Lençóis (2), Medicilândia (2), Miranguara (2), Monjolos (2), Mossoró (2), Nobres (2), Prados (2), Quixerê (2), Santa Maria da Vitória (2), Santa Teresa (2), São Gonçalo do Rio Abaixo (2), São Roque de Minas (2) — Aiuaba (1), Almirante Tamandaré (1), Altinopólis (1), Amapá (1), Analandia (1), Ananindeua (1), Apiai (1), Arcos (1), Aurora de Tocantins (1), Belém (1), Benfica (1), Bom Jesus da Lapa (1), Brasil Novo (1), Canálopis (1), Cantagalo (1), Cambuci (1), Conceição do Castelo (1), Capim Branco (1), Caraí (1), Catas Altas (1), Coronel José Dias (1), Corumbá de Goiás (1), Curaçá (1), Curvelândia (1), Diamantino (1), Dores de Guanhaes (1), Eldorado dos Carajás (1), Guarani de Goiás (1), Ipeuna (1), Ituaçu (1), Jaiba (1), Jandaira (1), Japaratuba (1), Jequitaí (1), Juquiá (1), Juvenilia (1), Lagarto (1), Lagoa da Confusão (1), Laranjeiras (1), Macapá (1), Maria da Vitória (1), Moeda (1), Montalvania (1), Mucugê (1), Natividade (1), Nova Redenção (1), Nova Roma (1), Ouro Preto (1), Ourolândia (1), Pau Brasil (1), Pedro Bernardo (1), Pedro Canário (1), Piumhi (1), Ponta Grossa (1), Porto Velho (1), Presidente Figueredo (1), Presidente Juscelino (1), Ribeirão Grande (1), Ribeira (1), Rio Pardo de Minas (1), Rosario do Oeste (1), Salvaterra (1), Santa Quitéria (1), Santa Maria de Itabira (1), São Francsco do Guaporé (1), São João da Lagoa (1), São João da Lapa (1), São João da Ponte (1), Ubajara (1), Unaí (1), Umburana (1) and Vespasiano (1).
MAP OF TROGLOBIC
Below is the distribution map of troglobic in Brazil. Cave s.s. restricted species are represented by black dots, and phreatic species by red dots. Each dot may correspond to more than one cave and/or municipality.

BRAZILIAN TROGLOBIC (BLACK DOTS) AND PHREATIC (RED DOTS) METAZOA
CAVE FUNGI IN BRAZIL
Cave fungi represent a diverse array of species that underwent speciation beyond their subterranean confines, providing several benefits to the biosystems they inhabit. 292 fungal genera belonging to six phyla (Ascomycota, Basidiomycota, Basidiobolomycota, Chytridiomycota, Mucoromycota, and Mortierellomycota) have been recorded, and a few operational taxonomic units have been identified as Rozellomycota and Kickxellomycota. Nearly half of the cave fungi known worldwide are found in only 30 caves in Brazil (José Prazeres, Fungal Biology Reviews, 2025).
ADDITIONAL STUDIES, SOURCES, AND REFERENCES
Some inventories result in confirmation of the absence of troglobic in some locations, such as Martins (RN, Araujo et al., Espeleo-Tema, 2017). Some works also maybe inconclusive in quantity. Ferreira, Zampaulo & Souza-Silva (Chapter Book, 2022) cites 50 spp. in the Pains karst, only 6 described until the publication of the article, suggesting 44 spp. new undescribed. As the article does not bring a listing by group, here it is not considered to accept these numbers. Simões et al. (Subterranean Biology, 2015) cites 33 troglobic/trogomorphic species in Minas Gerais state, however, without discriminating which are exact troglobic, we decided not to count these taxa.
Some studies address the troglobic fauna of the mesovoid shallow substratum in cangas of Minas Gerais (e.g. Sabach, L, et al, Scientific Reports, 2024), with some interesting records. However, we do not analyze or explore studies in mesovoid environments here.
Of all the phyla that evolved troglobic species, Brazil has representatives of all except Cnidaria, Syndermata and Nematoda. Lineages with troglobic unknown for troglobic in Brazil includes Cnidaria (Balkans), Themnocephalida (S Europe), Prorhyhchidae (South Africa), Nematoda (Colombia and Europe), Syndermata/Acanthocephala (U.S.A), Sabellida (NE Italy, Slovenia, Croatia, Bosnia & Hercegovina), Phyllodocida (SE Mexico and Caribbean, Canary Islands, Oman, Socotra, W. Australia, Papua New Guinea, New Zealand and Fiji), Branchiopoda (Spain, Romania, France, Slovenia, Bosnia and Herzegovina), Anaspidaceae (Australia and Tasmania), Thermosbaenacea (USA, Cuba, Puerto Rico, Italy, France, Thailand, Cambodia, W Australia), Mysida (several places worldwide), Decapoda/Caridea (widely worldwide), Decapoda/Astacida (Papua New Guinea, Cuba, Mexico, North America), Decapoda/Brachyura (widely worldwide), Insecta/Psocoptera (Venezuela), Insecta/Lepidoptera (Philippines, Hawaii and Colombia), Arachnida/Opilioacarida (Cuba, Thailand and Vietnam), Arachnida/Schizomida (Cuba, Jamaica, Belize, Mexico, USA, Ecuador, and Australia), Arachnida/Thelyphonida (Laos), Arachnida/Ricinulei (Venezuela, Belize, Cuba and Mexico) and Amphibia (USA and Balkans in Europe).






1 PHYLUM PORIFERA ‣ [2] lists only one cave sponge worldwide; however, three described species exist, all belonging to the family Spongillidae and considered obligatory subterranean: Eunapius subterraneus Sket & Velikonja, found in the Ogulin caves, Croatia; Arinosaster patriciae Volkmer-Ribeiro, Tavares-Frigo, Ribeiro & Bichuette, collected in the Parecis System, Mato Grosso state, C Brazil (Neotropical Biology and Conservation, 2021); and Racekiela cavernicola Volkmer-Ribeiro, Bichuette & Machado, collected in Lapa dos Brejões, northern Bahia state, NE Brazil (Volkmer et al., Neotropical Biology and Conservation, 2010). Both Brazilian troglobic species are corroborated by [6]. A fourth, yet undescribed species was discovered in central Mexico (Legendre et al., Subterranean Biology, 2023).


2 PHYLUM CNIDARIA ‣ many records of Cnidaria in freshwater caves can be found in Zagmaster et al. (Speleobiology, 2011), including in Mexico, USA, Australia, and several Central European countries. Velkovrhia enigmatica Matjašić & Sket, 1971 (Bougainvilliidae, Hydrozoa, PHOTO), from the Dinarides in the Balkan Peninsula, is the only known troglobic freshwater cnidarian. It has been reported from five caves, in Slovenia (3), Croatia (1), and Bosnia & Hercegovina (1), according to Magmajster (Natura Sloveniae, 2003). Unfortunately, it is not listed among freshwater Cnidaria in [3].
3 PHYLUM PLATYHELMINTHES ‣ according to our survey, six groups of flatworms include troglobic species, three of these exclusive from USA: Cestoda (Proteocephalus poulsoni Whitaker & Zober, 1978, from Kentucky), Trematoda (Brachycoelium longleyi Moravec & Huffman, 2000, from Texas, parasitic in Typhlomolge rathbuni Stejneger, Caudata, SEE), Alloeocoela (1, Prorhynchidae, Geocentrophora cavernicola Carpenter, 1970, from Kentucky, Virginia and West Virginia), Tricladida, Proseriata (1, South Africa, SEE), and Temnocephalida. At the species level, our data indicate 178 troglobic Platyhelminthes species worldwide, with 3 in Africa [7], 111 in Europe [2 | 21], 4 in Russia [21], 34 in North America [33], 6 in Mexico [5], 2 in Guatemala [31], 1 in Jamaica [24] and 17 in Brazil.
Tricladida in the U.S.A includes (3:4/)31 spp. in Dendrocoelidae (two in Dendrocoelopsis), Kenkiidae (21 in Kenkia and Sphalloplana), and Planariidae (8 in Phagocata).
For Tricladida and Temnocephalida in Western Europe, consolidated data are available only for the Balkans [29], along with a general reference [21, p936], which reports a total of 112 troglobic species in this region (99 Tricladida and 13 Temnocephalida). A record of a Temnocephalidan species associated with a crab in Papua New Guinea, previously considered troglobic, is here rejected [22]. No troglobic Platyhelminthes have been reported from China [2].
All known Brazilian troglobic Platyhelminthes belong to the order Tricladida [this work], spanning all three of its clades: Continenticola (13, Girardia, Dugesiidae, and one Geoplanidae), Maricola (1), and Cavernicola (2, VIDEO). Six undescribed species have also been cited, from Pará [PA/2], Mato Grosso do Sul [MS/1], São Paulo [SP/2], these Continenticola, and one Cavernicola for Rio Grande do Norte [RN/1], and one of undetermined order for Minas Gerais [MG/7]. Brazil is the only country with troglobic representatives in all three Tricladida clades. Sluysia triapertura Leal-Zanchet & Souza, 2018 represents the first Maricola triclad recorded from a freshwater cave habitat (Souza et al., Invertebrates Systematics, 2018).
UNDETERMINED ORDER/FAMILY


CAVERNICOLA


CONTINENTICOLA/DUGESIIDAE












CONTINENTICOLA/GEOPLANIDAE


MARICOLA


4 PHYLUM NEMATODA ‣ despite the extreme diversity of habitats they can occupy, in contrast with their marked morphological homogeneity, there is no systematic body of knowledge regarding troglobic Nematoda. Even basic criteria to define whether a species is troglobic do not exist. Although there are numerous collections, there is little precision about what truly constitutes a troglobic Nematoda. A careful review of Du Preez (Nematology, 2017) reveals that seven species are possibly true troglobic: Desmoscolex aquaedulcis Stammer, 1935 (Slovenia), Thalassoalaimus aquaedulcis Schneider, 1940 (Slovenia), Halalaimus stammeri Schneider, 1940 (Slovenia), Hemicycliophora aquatica Loos, 1948 (Belgium), Stenonchulus troglodytes Schneider, 1940 (Slovenia, Austria), Mylonchulus cavensis Schneider, 1940 (Hungary), and Chronogaster troglodytes Poinar & Sarbu, 1994 (Romania). Axonchium sbordonii Zullini, 1973, from Sima del Ojito, Chiapas, Mexico, is rejected in this work (Mexican Cave Fauna, 2022). In addition, one possibly troglobic species in Rhabditida was collected in Colombia [19 + notes]. Additionally, [33] lists two troglobic Nematoda in the USA: Amphibiocapillaria texensis Moravec & Huffman, 2000 (from Texas, parasitic in Typhlomolge rathbuni Stejneger, Caudata, SEE) and Rhabdochona longleyi Moravec & Huffman, 1988 (parasitic on Trogloglanis pattersoni and Satan eurystomus, both Ictaluridae, from the subterranean waters of Texas, SEE). Thus, a total of ten troglobic Nematoda are recognized in this text.
5 PHYLUM SYNDERMATA ‣ Syndermata is the current phylum that encompasses the former phyla Rotifera and Acanthocephala. To date, only one species in this group has been reported as troglobic: Dendronucleata americana Moravec & Huffman, 2000, a parasite Eoacanthocephala of Typhlomolge rathbuni Stejneger (Amphibia Caudata), known only from Texas, USA ([33] | Moravec & Huffman, Folia Parasitologica, 2000).
6 PHYLUM NEMERTEA ‣ according to [2], 3 out of 20 known freshwater Nemertea species are possibly troglobic (stygobiotic): Prostoma puteale Beauchamp, 1932, found in France, Switzerland, and Germany, and P. hercegovinense Tarman, 1961, found in Bosnia & Herzegovina. Additionally, one species has also been described from New Zealand. In Brazil, there are three possibly troglobic Nemertea: one from the Alto do Ribeira region in São Paulo state [SP/2], one from central Minas Gerais state [MG/1], and one from Padre Cave in Bahia state [BA/7]. If confirmed, these would be the first troglobic Nemertea described from the New World.




7 PHYLUM ANELLIDA ‣ based on our surveys, three clades within Annelida has troglobic: Sabellida, Phyllodocida and Clitellata. Some families have adapted to living in the water column of marine/anchialine caves, including the normally benthic Protodrilidae, Nerillidae, and Scalibregmatidae. The aptly named genus Troglochaetus (Nerillidae) is also found in the hyporheic zone of caves, wells and springs. Troglochaetus has rudimentary parapodial cirri and, like all nerillids, development is direct, either via anexternal brood or in cocoons (Galsby et al., Diversity, 2021). Numerous marine species live in conditions similar to cave conditions: inside the bottom material, under stones and in the rock fissures. Morphological adaptations to cave environment such as the absence of body pigmentation and lack of eyes are rarely observed in polychaetes; principally therefore they can be classified as stygobionts only on the basis of their habitat [2].
Our survey point to 174 known troglobic Annelida, 159 in Clitellata, one in Sabellida, and 14 in Nemanereis.
SABELLIDA
Sabellida has one full troglobic, Marifugia cavatica Absolon & Hrabe, 1930 (Serpulidae), a eyeless species known from caves situated in Dinaric Karst in NW Italy, Slovenia, Croatia, and Bosnia & Hercegovina. Even though some localities are situated few kilometers from the seashore this species has never been found in brackish or salt water [2].
PHYLLODOCIDA
Phyllodocida has at least 14 cavernicolous (however, all inconcluse be troglobic, C. J. Glasby et al., ZJLS, 2014) species representing genus Namanereis (Nereididae), in Guerrero state in SE Mexico, Cuba, Hispaniola and Saint Vicent (1, N. cavernicola Solís-Weiss & Espinasa, 1991 — some populations extreme continental, found in cave pools above 1600 m asl and over 170 km from the coastline), exclusive from Caribbean (5, SEE), Canary Is. (2, SEE), Oman (1, SEE), Socotra (2, SEE), W Australia (1, SEE), Papua New Guinea (1, SEE), New Zealand and Fiji (1, N. tiriteae Winterbourn, dubious troglobic status, SEE).
CLITELLATA
Aquatic members have a habitus that makes them pre-adapted to live in the subterranean environment. In addition, they do not exhibit any peculiar morphological adaptations to subterranean life that can be seen in other subterranean organisms (loss of eyes, elongation of appendages and body, loss of pigmentation, increase in sensory structure). Therefore, stygobitic nature in Clitellata can only be inferred from their exclusive presence in the subterranean environment. Despite their morphological pre-adaptation, few aquatic Clitellata are exclusively present in groundwater.
Our survey listed 159 troglobic species of Clitellata worldwide, in Guinea (2), Morocco (1), Europe (100), Oman (2), Iran (1), Japan (2), China (2), New Zealand (2), Bermuda (1), Cuba (5), Haiti (2), USA/Canada (22), Mexico (4), Venezuela (1), Ecuador (2) and Brazil (10). These 159, 12 unplaced at family: two in Mexico and all Brazilian.








ENCHYTRAEIDAE
(6/)12 troglobic spp. in caves, 10 in Europe (Poland, Hungary, Romania, Greece) and two in Ecuador: Marionina ecuadoriensis Righi and Hemienchytraeus mauriliae Righi.
HAPLOTAXIDAE
(3/)9 troglobic spp., 8 cited in [20] in Europe (5, Spain, France and Bulgary), Nippon (1) and Guinea (2), and Haplotaxis brinkhursti Cook, 1975 added for USA in [25] and [33], in West Virginia.
LUMBRICIDAE
(2/)2 spp. (Eophila hypogea Malevics, 1947 and Archaeodrilus cavaticus Czerniavsky, 1880), cited for Georgia and Abkhazia [21].
LUMBRICULIDA (includes Dorydrillidae)
(9/)45 troglobic spp., 31 by [20] for West Europe (29, UK, Spain, France, Belgium, Germany, Switzerland, Italy, Estonia, Slovenia, Czech Republic, Croatia, Serbia, Bosnia-Herzegovina, Macedonia, Romania), Abkhazia (undescribed species, counted here as only one, are known from Abkhazia [21]), and Japan (1), plus (5/)14 troglobic spp. from USA in Virginia, Alabama, Tennessee in E, Oregon and California, by [33].
PARVIDRILLA
Two troglobic spp. in Parvidrilus, one in Italy and Slovenia, and Parvidrilus strayeri Erseus, 1999 in Alabama, USA ([33], SEE).
PHREODRILIDAE
(3/)5 troglobic spp., in New Zealand (1), Oman (2), Morocco (1) and Australia (1).
TUBIFICIDAE
(19/)53 troglobic spp., nine spp. in New World (Phallodriloides macmasterae Erséus, 1986 in Bermuda, four Pristina and Clitellio cavernicolus Botea, 1983 in Cuba, Dero haitiensis Dumnicka, 1986 and Spirospermoides stocki Dumnicka 1983 in Haiti and adjacent islands, and Krenedrilus papillatus Dumnicka, 1983 in Venezuela); 39 in Europe (Portugal, Spain, France, Germany, Switzerland, Italy, Poland, Czech Republic, Slovenia, Macedonia, Greece and Turkyie); and 5 more species, from Russia, Uzbekisthan, Abkhazia and Georgia [21]. Gianius aquaedulcis Hrabě, 1960 is rejected in USA/New York (SEE).
HIRUDINA
At least 10 spp. of troglobic leeches are listed, in three families (five in Europe and former USSR, one in Iran, two in China, two in Brazil): Haemopidae, Haemadipsidae and Erpobdellidae, usually exhibiting two specific morphological features, namely milky-white coloration and lack of eyes or eye pigment (Cichocka et al., Zootaxa, 2015). Two possibly undescribed troglobic species was collected in C Minas Gerais state [MG/1] and [MG/7], Brazil. If confirmed, they will be the firsts troglobic Hirudinea described for the New World.
Described troglobic leeches includes Haemopis caeca Klemm & Sarbu, 1998 from Dobrogea in Romania (Haemopidae), Erpobdella absoloni Johansson, 1913 (sometimes as Dina absoloni) in N Italy–Balkans–Turkey–Georgia [21], Erpobdella underminated spp. from Abkhazia [21], E. krasensis Sket, 1968, and E. mestrovi Kerovec, Kučinić & Jalžić, 1999 from Croatia (Cichoka, 2015), E. borisi Cichocka & Bielecki from NW Iran, and two in China. Possibly undescribed species, probably Erpobdellidae, occur in caves of China and the USA (Sket & Trontelj, Hydrobiologia, 2006), non counted here. Two known species of Haemadipsidae, Leiobdella jawarerensis Richardson, 1974 possess five pairs of eyes, which is the only pigmented part on its body, inhabiting the aphotic zone of a caves in Papua New Guinea (Cichoka, 2015), however is no accepted here as troglobic.


BRANCHIOBDELLIDA (crayfishworms)
Stygobitic, usually ectoparasites of crustaceans, some of them (similarly to flatworms Themnocephalida) were found on one to three host species only [2]. At least 8 troglobic, two in Mexico (Mexican Cave Fauna, 2022) and six in USA [33], all Cambarincola in Alabama, Florida, Indiana, Kentucky and Tennessee.
8.1 MOLLUSCA/BIVALVIA ‣ typical troglobic mollusks usually exhibit the following characteristics: (1) absence or reduction of pigmentation, (2) absence or reduction of eyes, and (3) some degree of shell size reduction (smaller than their epigean relatives). Troglobic species are found in both non-exclusive marine Mollusca classes. Bivalvia includes five described troglobic species worldwide: three confirmed Euglesa (Euglesidae) from Eastern Europe [21] — in Russia (1), Abkhazia (1), and Georgia (1); Congeria kusceri Bole, 1962 (Dreissenidae, Total Croatia News), known only from Slovenia to SW Bosnia and S Dalmatia; and Eupera troglobia Simone & R. L. Ferreira, 2022 (Sphaeriidae), restricted to western Tocantins state in central Brazil, representing the only known troglobic Bivalvia in the Western Hemisphere.


8.2 MOLLUSCA/GASTROPODA ‣ troglobiont (terrestrial) snails are all pulmonates, except for the prosobranch Pholeoteras euthrix Sturany, 1904, known from caves in southern Croatia, southern Herzegovina and Greece (ANIMALBASE). All stygobitic Gastropoda are prosobranchs except Acroloxus tetensi Kušcer, 1832 from the Dinaric Mountains in Slovenia, and Hydrophrea from New Zealand, which are pulmonates.
Our survey will reliably point to at least 491 known species of troglobic Gastropoda: 368 in Europe, 16 in Africa, 22 in former USSR [21], one in Iran [8 | 9 | 10], two in China [2], one in New Guinea [22], four in Australia [12], one in New Zealand [2], 39 in USA/Canada [33], 9 in Mexico [18] and 28 in Brazil.
Recent data point to (5:20/)39 spp. for aquatic members in USA/Mexico, by Gladstone et al. (Conservation Biology, 2022). USA has (6:17/)39 spp. of troglobic Gastropoda in Basommatophora (2:2/2), Stylommatophora (2:2/4, in Helicodiscus and Glyphyalinia), and Littorinimorpha (2:13/33).
There is no exact data on the diversity of troglobic Gastropoda in Western Europe. Here, we will adopt artifically the number of 368, which is the sum of 143 stygobites from France [2], 169 stygobites from the Balkans (Falniowsk, A. et al., Hydrobiologia, 2021), and 56 troglobiontes mentioned in [2]. Europe is home to 11 genera of strictly troglobic snails: Troglaegopis, Sciocochlea, Speleodentorcula, and Spelaeoconcha (all monotypics), Paraegopis (5, 1 TB), Argna (6, 1 TB), Klemmia (2, 1 TB), Cryptazeca (4, 2 TB), Virpazaria (7, 3 TB), Spelaeodiscus (10, 9 TB) and Zospeum (35 TB), mainly in the Dinaric karst.
Here we accept 28 names as troglobic in Brazil: six already described and mentioned expressly in R.L.Ferreira et al. (Zoologia, 2023), two describeds listed in Salvador & Bichuette (Zoosystematics and Evolution, 2024), and 20 not yet described: 6 Potamolithus in São Paulo [SP/1], 3 in Pará [PA/2], 1 in Paraná [PR/1], 2 in Mato Grosso do Sul [MS/1] and 8 in Bahia [BA/2][BA/3][BA/4][BA/7]. Overall, Brazil has at least nine genera and nine families.
Cavallaria et al. (Zoologia, 2022) cites 19 described troglobic gastropoda from Brazil, however, troglobic status for many of them are rejected by several autors.
UNDETERMINED FAMILIES






CAENOGASTROPODA
DIPLOMMATINIDAE


EUPULMONATA
CHAROPIDAE (STYLOMMATOPHORA)


COCHLIOPIDAE (LITTORININOMORPHA)




GASTROCOPTIDAE (STYLOMMATOPHORA)


LITHOGLYPHIDAE (LITTORININOMORPHA)







POMATIOPSIDAE (LITTORININOMORPHA)


SCOLODONTIDAE (STYLOMMATOPHORA)


SYSTROPHIIDAE (STYLOMMATOPHORA)




TOMICHIIDAE (LITTORININOMORPHA)




9 PHYLUM ONYCHOPHORA ‣ only two troglobic Onychophora species are officially recognized: Peripatopsis alba Lawrence, 1931 (Peripatopsidae) from South Africa, and Speleoperipatus spelaeus Peck, 1975 (Peripatidae) from Jamaica [24 | SEE], with a possible additional species in the Galapagos, Ecuador. Unofficially, there is also a troglobic species in Brazil, not yet formally described, found in caves of the Bodoquena System, Mato Grosso do Sul state, which may even represent a new genus [MS/1].
PERIPATIDAE


10.1 ARTHROPODA/ARACHNIDA ‣ all orders of Archnida has troglobic except Holothrida and Solifugae. 337 troglobic Arachnida occur in North America (in Acari, Pseudoscorpiones, Araneae, Opiliones, Schizomida, Scorpionida), 144 in China [2], 184 in Mexico [5], and 178 in Brazil.
Among Acari, two superorders and four orders contain troglobic species: Parasitiformes with the orders Opilioacarida and Gamasida, and Acariformes with the orders Sarcoptiformes and Trombidiformes. Despite the extreme diversity of mites in caves in Brazil, with 126 families collected in caves in the country [6], only one spp. is formally described, and another 7 are identified as troglobic without description, in Bahia [BA/1][BA/3], Minas Gerais [MG/1][MG/4] and Mato Grosso do Sul [MS/1]. At least five Acari are troglobites in Western Europe, in Belgium (4, SEE) and Italy (4, SEE | SEE), and 4 in Russia [21], 12 in Cuba [13], 1 in Bolivia [15], (10:17/)26 in USA/Canada [2 | 13 in Rhagidiidae], 9 in Venezuela [17], 2 in Mexico [18], 4 in SE Asia [22], and 2 in America Central [23].
OPILIOACARIDA ‣ although several species in Brazil are associated with subterranean habitats, none are classified as troglobic (Berbardi & Borges-Filho, Subterranean Biology, 2018). Troglobic worldwide occur only in Cuba, Thailand and Vietnam [4].
IXODIDA ‣ Galán & Herrera (BSVE, 2006) lists Antricola silvai Cerny, 1967 from Curazao maybe a troglobic. If confirmed, it will be the only record of an Ixodida troglobic worldwide. Huge populations occur in caves in Brazil, associated with bats, all troglophiles.
MESOSTIGMATA ‣ in Mesostigmata, a large number of cave species are permanent or temporary ectoparasites of bats, but most are free living. Three troglobic undescribed species in Brazil, collected in Minas Gerais [MG/1] and Bahia [BA/3] states. In USA occur 5 spp. of troglobic Mesostigmata in Laelapidae (1/1, Kentucky), Macrochelidae (1/2, Kentucky) and Veigaiidae (1/2, Indiana), by [33].
UNPLACED FAMILY


DITHINOZERCONIDAE


PACHYLAEPIDAE


TROMBIDIFORMES ‣ Prostigmata includes a few poorly known cave species in the families Leeuwenhoekiidae, Trombiculidae, and Trombidiidae, frequent on or close to guano accumulations intropical caves, and bat or invertebrate parasites at larval stage. Proterorhagidiidae has a single relictual troglobic, Proterorhagia oztotloica Lindquist & Palacios-Vargas, 1991 from Mexico. Troglobic Rhagidiidae are often linked to cold caves in the Holarctic zone [4]. One troglobic Rhagidiidae also occurs in Mexico. In Brazil occur a single described and four undescribed species in Pará [PA/2], Bahia [BA/1] and Minas Gerais [MG/4] states. Venezuela has eight troglobic Hydracnidia in three families (Galán & Herrera, BSVE, 2006). One troglobic occur in Africa [7], two in America Central [23] and one in Bolivia [15]. In USA occur 25 troglobic spp. in 7 families: Aturidae (1/1, Florida and Texas), Bogatiidae (1/1, Illinois), Cocceupodidae (1/1, Kansas), Hydryphantidae (1/1, Texas), Hygrobatidae (1/3), Limnesiidae (1/1, Texas), Limnohalacaridae (2/3, all in Indiana), Nudomideopsidae (1/1, Texas), and Rhagidiidae (7/13, widely distributed), by [33].
UNDETERMINED FAMILY


ERYTHRAEIDAE


LABIDOSTOMATIDAE


RHAGIDIIDAE


SPHAEROLICHIDAE


SARCOPTIFORMES (ORIBATIDA) ‣ troglobic species of this group are surprisingly rare: some species of Schwiebea (Acaridida) from humid hypogean habitats, devoid of adaptations to subterranean life, and a few European Belbidae, such as Metabelbella phalangioides are pale and long-legged [4]. Brazil has two undescribes species in Mato Grosso do Sul [MS/1] and Bahia [BA/3] states. In USA occur two species of troglobic Sarcoptiformes, both from Kentucky: Belba bulbipedata (Packard, 1888) in Belbidae, and Galumna alata (Hermann, 1804) in Galumnidae, by [33].
HIDROZETIDAE


OEHSERCHESTIDAE


PSEUDOSCORPIONES ‣ a total of 440 troglobic Pseudoscorpiones species worldwide in 15 families are included here: 132 in Europe [29] — exclusive Balkans numbers, 6 in Africa [7], 3 in Russia, 2 in Georgia [21], 40 in China [2], 7 in Korea (SEE), 4 in SE Asia to New Guines [22], (8:27/)153 in USA/Canada [33], (5:)40 in Mexico [18], 1 in Guatemala [31], 3 in Cuba [13], 4 in Jamaica [24], 1 in Aruba (SEE), and (6:)49 in Brazil, being 21 undescribed, in Pará [PA/2], Rio Grande do Norte [RN/1], Bahia [BA/2][BA/3][BA/4], Minas Gerais [MG/1][MG/6][MG/10], São Paulo [SP/3], Paraná [PR/1] and Goiás [GO/1] states. USA has (8:)153 spp. [33], in Bochicidae (1/1, Texas), Chernetidae (6/12), Chthoniidae (7/97, mainly in Alabama), Ideoroncidae (1/1, Arizona), Larcidae (1/5, California, Arizona and Texas), Neobisiidae (8/27, mainly in Texas), Pseudogarypidae (1/3, Arizona and California) and Syarinidae (2/7).
BOCHICHIDAE






CHTHONIIDAE




























CHERNETIDAE












IDEORONCIDAE


OLPIIDAE


SYARINIDAE


ARANEAE ‣ 705 troglobic species worldwide in 48 families: 194 in Europe (SEE), 21 in Africa, 1 in Iran [8 | 9 | 10], (16:30/)104 in China [2], 14 in SE Asia to New Guinea [21], 101 in Australia [2 | 12, extrapolative], (8:23/)121 in USA/Canada [33], (11:)59 in Mexico [18], 1 in Guatemala, 7 in Cuba [13], 9 in Jamaica [24], 3 in Colombia [19] and 69 in Brazil.
USA has (8:23/)121 spp. in Dictynidae (52, all in Cicurina from Texas except one in Alabama), Leptonetidae (4-5/25, mainly in Texas), Linyphiidae (9/15, widely distributed), Lycosidae (2/2, both in Hawaii), Nesticidae (2/21, mainly in Tenessee and Texas), Ochyroceratidae (2/2, California and Hawaii), Tengellidae (2/3), and Theridiidae (1/1, Arizona), by [33]. Colombia includes three troglobic spiders (in three families, Pisauridae without Brazilian troglobic) by [19]. Brazil has (16:)81 spp., 46 of thesse undescribed in Pará [PA/2], Tocantins [TO/1], Rio Grande do Norte [RN/1], Bahia [BA/1][BA/2][BA/3][BA/4][BA/5][BA/6][BA/7], Minas Gerais [MG/1][MG/3][MG/5][MG/10], Espírito Santo [ES/1], São Paulo [SP/2], Mato Grosso do Sul [MS/1] and Paraná [PR/1] states.
Among Theraphosidae, troglobitc species includes Orphnaecus pellitus Simon, 1892 from Philippines (SEE), six Hemirrhagus from Mexico, and Tmesiphantes hypogaeus Bertani, Bichuette & Pedroso, 2013 from Brazil (Bertane, Bichuette & Pedroso, Anais da Acadêmia Brasileira de Ciências, 2013). Besides several collections inside caves, seven spp. of Ochyrocera describeds in Brescovit et al. (Zootaxa, 2018) and three Speocera describeds in A.D. Brescovit et al. (Annales de la Société entomologique de France, 2023) are not troglobic.
UNDETERMINED FAMILY




CAPONIIDAE






CTENIIDAE


DIPLURIDAE


HAHNIIDAE


MICROSTIGMATIDAE


NESTICIDAE


OCHYROCERATIDAE





















OONOPIDAE








PALPIMANIDAE


PHOLCIDAE




PRODIDOMIDAE














SICARIIDAE




SYMPHYTOGNATHIDAE


TELEMIDAE


TETRABLEMMIDAE








THERAPHOSIDAE


OPILIONES ‣ four order In Cyphophthalmi the unique New World troglobic is Neogovea mexasca Shear, 1977 from Mexico. Eupnoi no has true troglobic. Dyspnoi (Palpatores) has only one troglobic species in New World, Ortholasma sbordonii Šilhavý, 1973 in Mexico. All remaining troglobitc opiliones in New World belongs Laniatores [2 | 4].
Worldwide 144 troglobic Opiliones occur in Balkans in Europe (21), Africa and Madagascar (2), Russia and adjacent countries (6), SE Asia to New Guinea (3), USA (7:12/46), Mexico (4:17, SEE), Cuba (4), Jamaica (2), Venezuela (4, all in Trinella in Agoristenidae), Colombia (3) and Brazil (36). Brazil has (8:)43 spp., all Laniatores, 28 of these undescribed, in Pará [PA/2], Bahia [BA/2][BA/3][BA/7], Minas Gerais [MG/1][MG/3][MG/6], Espírito Santo [ES/1], Mato Grosso do Sul [MS/1], Pará [PA/1][PA/2], Rio Grande do Norte [RN/1] and Paraná [PR/1] states. USA has (7:12/)46 spp. in Cladonychiidae (1/2, Idaho), Paranonychidae (1/7), Phalangodidae (6/26, mainly Texas and California), Sabaconidae (1/3), Stygnopsidae (1/2, Texas), Taracidae (1/5, California, Oregon and Nevada), and Travuniidae (1/1, Washington), by [33].
UNDETERMINATE FAMILY




COSMETIDAE


CRYPTOGEOBIIDAE




ESCADABIIDAE














GERDESIIDAE


GONYLEPTIDAE


















KUMULIDAE


TRICOMMATIDAE


ZALMOXIDAE


AMBLYPYGI ‣ troglobic in Amblypygi (28), are cited in four families, three in New World: Charinidae, with Charinus in New World, including all 11 Brazilian troglobic and two in Venezuela (Galán & Herrera, BSVE, 2006), without troglobic in Mexico; Paracharontidae, with Jorottui, a monogeneric troglobic endemic to N Colombia (Novataxa, 2023); and Phrynidae, with true troglobic in Mexico (11), Cuba (1) and Colombia (1, Angarita-Sierra, CCE/2018). No troglobic Amblypygi occur in USA Worldwide, one troglobic occur in Africa [7].
CHARINIDAE











SCHIZOMIDA ‣ both two families of this order has troglobic. Hubbardiidae known from caves in Cuba, Jamaica, Belize, Mexico (14), California (Hubbardia shoshonensis Briggs & Hom, 1972, USA, [33]), Ecuador, Australia, and Brazil, with a single undescribed spcies from Pará state [PA/2]. In Protoschizomidae, all 10 spp. are troglobic, nine in Mexico and Agastoschizomus texanus Monjarez-Ruedas et al., 2016 in Texas, U.S.A [33].
HUBBARDIIDAE


THELIPHONIDA ‣ no confirmed troglobic were reported in this order, but Typopeltis magnificus Haupt possibly troglobic from Laos [4], and accepted here.
PALPIGRADI ‣ troglobic Palpigrada occur in Europe (23, SEE), Thailand (4), Cuba (1), Indonesia (2), and Brazil (30). All genera in this order except Leptokoenenia have troglobic [2]. In New World, troglobic Palpigrada occur only in Brazil (two Allokoenenia, 25 Eukoenenia, three undeterminate genus) and Cuba, with possibly undescribed species in California [2] and Mexico [4]. 12 undescribed in Brazil, in Minas Gerais [MG/1], Bahia [BA/1][BA/2][BA/3][BA/6][BA/7], São Paulo [SP/2] and Paraná [PR/1].
EUKOENENIDAE






























RICINULEI ‣ troglobic in Pseudocellus have been described from Cuba (1), Belize (1), Mexico (11) and Venezuela (1, SEE). Troglobic Ricinulei does not occur in Brazil.
SCORPIONIDA ‣ there are 33 troglobic scorpions worldwide, in Indonesia (1, SEE), Malaysia (1, SEE), Montenegro (1, SEE), Israel (1, Akrav israchanani, SEE), Madagascar (2, SEE), Laos [22], Vietnam [22], Australia (2, SEE | SEE), USA in California (1, Uroctonus grahami Gertsch & Soleglad, 1972, Vaejovidae), Mexico (13, SEE, excludes two from leaf litter), Cuba (2, SEE), Bahamas (1, SEE), Dominican Republic (2, SEE), Ecuador (Troglotayosicus vachoni Lourenço, 1981, Troglotayosicidae), Venezuela (Taurepania trezzii Vignoli & Kovařík, 2003, Chactidae) by [19] and Brazil (2, Buthidae, both in Bahia state). Additional species known only from caves show slight reduction in pigmentation or eyes but are probably not troglobic [2].
BUTHIDAE


10.2 PAUROPODA ‣ no described troglobic Pauropoda are currently known [2]. However, one undescribed species was collected in Minas Gerais [MG/1], SE Brazil. If confirmed, it would be the first troglobic Pauropoda species described worldwide.


10.3 SYMPHYLA ‣ only three species of this order are described as troglobic: one in Tasmania (Hanseniella magna Scheller, SEE) and two in Slovenia. Three additional species have been collected in Brazil — one in the Alto do Ribeira karst, São Paulo state [SP/3], one in Minas Gerais [MG/6], and one in Padre Cave, Bahia [BA/7] — but remain undescribed. If confirmed as troglobic, they would represent the first Symphyla troglobites described from the New World.




10.4 DIPLOPODA ‣ the figures presented here indicate 655 troglobic Diplopoda species, although this estimate is highly imprecise — particularly due to the uneven sampling effort and high diversity in Europe: 191 in Europe [29 | Iberian Peninsula | Romania], 11 in Russia and adjacent former Soviet republics [21], 5 in Africa [7], 1 in Iran [8 | 9 | 10], 114 in China [2], 15 from SE Asia to New Guinea [22], 19 in Australia [12], 133 in the USA and Canada [2 | in five orders], 73 in Mexico [5 | in six orders], 6 in Colombia [19], 2 in America Central [23 | 31 | Guatemala], 1 in Venezuela [17], 1 in Argentina (SEE), and 94 in Brazil (in 5 orders and at least 15 families, 81 undescribed).
UNDETERMINATE ORDERS


POLYXENIDA ‣ five troglobic worldwide, three described: Lophoproctus pagesi Condé, 1981 from Balearic Islands, Lophoturus speophilus Nguyen Duy-Jacquemin, 2014 and L. humphreysi Nguyen Duy-Jacaquemin, 2014 from Christmas Island [2], and six undescribed species was collected in Pará [PA/2], Bahia [BA/4] and Minas Gerais [MG/7] states in Brazil.
HYPOXEGENIDAE


LOPHOPROCTIDAE




POLYXENIDAE


GLOMERIDA ‣ troglobic in this order occur in Europe, China, two in SE Asia [22], Mexico [5 | six microphthalmic troglobic in Glomeroides], and America Central. 26 troglobic are collected in Old World.
SIPHONOPHORIDA ‣ possibly only three troglobic worldwide: one in Mexico, one in Texas (Siphonophora texascolens Chamberlin & Mulaik, 1941) and one in California, USA (Illacme tobini Marek et al., 2016) by [33], and one in Brazil, undescribed in Bahia state [BA/5].
SIPHONOPHORIDAE


GLOMERIDESMIDA ‣ a smaller order, with only three troglobitc species, Glomeridesmus sbordonii Shear, 1974 from Mexico [2 | 4], G. spelaeus Iniesta & Wesener, 2012 and three undescribed Glomeridesmus [PA/1][PA/2], all from Pará state, in N Brazil (Iniesta, Zootaxa, 2012 | SEE).
GLOMERIDESMIDAE




JULIDA ‣ dominant millipede order in Europe and many troglobic have been described. There are two troglobic species in U.S.A (both Zosteractinidae, from Alabama and Georgia) by [33], but relatively few in Asia, and none are known from Mexico [2 | 4].
SPIROBOLIDA ‣ this order has at least three troglobic: Reddellobus troglobius Causey, 1975 in Mexico, Speleostrophus nesiotes Hoffman, 1994, depigmented and eyeless, in Australia [2 | 4], and one undescribed in Colombia [19 | Rhinocricidae].
CALLIPODIDA ‣ troglobic species in this order are found in USA (two Tetracion, Abacionidae, from Alabama and Tennessee) by [33], China, 7 in SE Asia [22], one in New Guinea [22]. Unknown in Mexico [2 | 4].
SPIROSTREPTIDA ‣ this order is subdivided into Cambalidea and Spirostreptidea. Cambalidea includes 8 troglobic in New World: Cambala speobia Chamberlin, 1953 from gypsite caves in Texas (USA) by [33], and (2:3/)7 in Mexico (Iniesta & Ferreira, Zootaxa, 2013). One troglobic occur in Africa [3], one in Iran [9, in Cambalidae] and one in Colombia [19, Spirostreptidae]. In Brazil all troglobitc are Pseudonannolene (Spirostreptidea). Six undescribed species occur in caves from Goiás [GO/1], Espírito Santo [ES/1] and Bahia [BA/2] states.
PSEUDONANNOLENIDAE










CHORDEUMATIDA ‣ the most important millipede order in north temperate zone caves is the Chordeumatida. USA has (3:8/)108 spp., in Caseyidae (2/3, California and Oregon), Cleidogonidae (62 spp. in Pseudotremia, widely distributed), Conotylidae (7/12), Striariidae (2/3, California), Tingupidae (2/2, Colorado and Illinois), and Trichopetalidae (6/26, mainly Alabama and Arkansas), by [33]. One troglobic occur in Africa [3]. (2:2/)13 troglobic in Mexico [5]. Heterocaucaseuma deprofundum Antic & Reboleira is a troglobic from deep caves in the Abkhazia (a unrecognized state), and at 1,980m below the surface is the deepest occurring known millipede and perhaps the deepest occurring terrestrial arthropod worldwide (SEE).
STEMMIULIDA ‣ besides some cave-dwelling species (e.g. Stemmiulus in N Brazil), possibly only one species is assignated as troglobic, from Colombia [19].
POLYDESMIDA ‣ distributed worldwide, being 3 troglobic in Africa [7], 5 in SE Asia and New Guinea [22], 45 in Mexico [5], one in America Central [23 | Calymmodesmus in Guatemala], four in Colombia [19 | 3 in Chelodesmidae, 1 in Pyrodesmidae], one in Venezuela [17 | Trichopolydesmidae] and 61 in Brazil, 47 undescribed, in Pará [PA/1][PA/2], Rio Grande do Norte [RN/1], Bahia [BA/1][BA/2][BA/3][BA/4][BA/6][BA/6], Minas Gerais [MG/1][MG/3][MG/5][MG/6][MG/10], Espírito Santo [ES/1], São Paulo [SP/2][SP/3][SP/4], Paraná [PR/3], Goiás [GO/1] and Mato Grosso do Sul [MS/1]. USA has (3:8/)25 spp. troglobic in tbis order, in Macrosternodesmidae (6/18, mainly in W North America), Nearctodesmidae (1/1, Ergodesmus remingtoni (Hoffman, 1962), from Illinois) and Polydesmidae (1/6, all from Texas), by [33].
UNPLACED AT FAMILY LEVEL






CHELODESMIDAE










CRYPTODESMIDAE






DOBRODESMIDAE


FUHRMANNODESMIDAE




ONISCODESMIDAE









PARADOXOSOMATIDAE










POLYDESMIDAE






PYRGODESMIDAE
















SPHAERODESMIDAE


TRICHOPOLYDESMIDAE





10.5 CHILOPODA ‣ of the five orders of Chilopoda, four have cave species, all of them in Brazil. For cave Chilopoda worldwide, see Chagas and Bichuette (Zookeys, 2018). 55 troglobic occur in Europe [29 | 50 Lithobiidae, Cryptopidae in Spain and Italy, and Geophilidae in France and Croatia], 4 in USA [33 | in Lithobiomorpha and Cryptopidae], (2:)5 in Mexico [5 | two Lithobiidae, 3 in Newportia], 2 in Africa [7 | Lithobiomorpha and Scolopendromorpha one each], 1 in China [2], 1 in Panama [23], 1 in Colombia [19], and 23 in Brazil (13 Scolopendromorpha, 7 Geophilomorpha, two Lithobiomorpha, one Scutigeromorpha). All six families with troglobic has species in Brazilian caves except Balophilidae [4].
SCUTIGEROMORPHA ‣ one Sphendononema sp. collected in Andaraí municipality, center Bahia state [BA/3] in Brazil representes the unique troglobic Scutigeromorpha worldwide.
PSELLIOLIDAE


LITHOBIOMORPHA ‣ following [2], about 50 troglobic species of this order (all Lithobiidae) occur in many countries in Europe, one in the Maghreb [7], two in North America (Nampabius turbator Crabill, 1952 from Virginia and Stenophilus californicus (Chamberlin, 1930) from California), two in Mexico, and remaining from E Asia. Two undescribed species was collected in Bahia [BA/2] and Rio Grande do Norte [RN/1] states in Brazil.


SCOLOPENDROMORPHA ‣ five families in this order, three of them has troglobic: Scolopendridae: Otostigmus cooperi (Panama); Cryptopidae: Theatops phanus Chamberlin, 1951 (Texas, USA), Thalkethops grallathrix Crabill, 1960 (New Mexico, USA), Cryptops longicornis (Spain), C. vulcanicus (Canaries, Spain), C. umbricus (Italy), C. roeplainsensis, C. camoowealensis (Australia), C cavernicolus, C. troglobius (Cuba), C. iporangensis, C. didi (SE Brazil) and C. spelaeoraptor (Bahia, Brazil); and Scolocryptopidae: Newportia leptotarsis (Cuba), three in Mexico, N. stoevi (Puerto Rico), N. spelaea (Bahia, Brazil), N. potiguar (Rio Grande do Norte, Brazil), Scolopocryptops troglocaudatus (Bahia, Brazil), and Scolocryptops sp. in Colombia, [9]. Three undescribed species occur in Brazil in Cryptopidae, in Pará [PA/2] and Bahia states [BA/2][BA/3], and 4 in Scolocryptopidae, in Rio Grande do Norte state [RN/1]. One troglobic Scolopendromorpha occur in mainland Africa [7 | unknown origin].
CRYPTOPIDAE






SCOLOCRYPTOPIDAE








GEOPHILOMORPHA ‣ our survey identified 11 troglobic Geophilomorpha species worldwide, of which 5 have been formally described (Nunes et al., Zootaxa, 2019): Plutogeophilus jurupariquibara (Brazil), Geophilus persephones (France) and G. hadesi (Croatia) in Geophilidae; Ityphylus cavernicolus (Cuba) in Ballophilidae; and Schendylops janelao (known only from nortern Minas Gerais state, Brazil) in Schendylidae. Five undescribed species occur in Bahia [BA/1][BA/2], São Paulo [SP/3], Rio Grande do Norte [RN/1] and Minas Gerais [MG/5] in Brazil.
GEOPHILOMORPHA UNDETERMINED FAMILY




GEOPHILIDAE


SCHENDYLIDAE


10.6 OSTRACODA ‣ although information is limited, it is estimated that approximately 600 species are stygobitic [2, p. 334], however our numbers indicate only 227, being 114 in Europe [30], 4 in Africa [7], 5 in Russia and adjacent countries [21], 74 in Australia [12], (2:14/)22 in the USA [33 | all Podocopida, highly centered in Texas], (2:2/)3 in Mexico [5 | excludes anchialine Danielopolina mexicana Kornicker & Iliffe], 5 in Cuba [13], 3 in Jamaica [24], 2 in Venezuela [17 | Cyprididae], and 1 in Brazil, a undescribed species collected in Rio Grande do Norte [RN/1] state, and none from China.


10.7 COPEPODA ‣ our survey identifies 809 troglobic copepods (all stygobitic) worldwide, with 547 recorded in Europe [30], 83 in Australia [12], 61 in Africa [7], 30 in Russia and neighboring countries [21], 23 in China [2], 18 in Cuba [13], (5:7/)18 in the USA [33 | 15 Cyclopidae, 9 Diacyclops, mainly in Indiana and Tennessee], 12 in Iran [9 | 10 | 11], 12 in Mexico [5], and 5 in Brazil, all undescribed, in Rio Grande do Norte [RN/1], Bahia [BA/7], Minas Gerais [MG/6] and São Paulo [SP/3]. Venezuela includes one troglobic Copepoda, in Cyclopidae [17].
UNKNOWN GROUP




HARPACTICOIDEAE


CYCLOPOIDEA/ENTOBIIDAE


10.8 MALACOSTRACA ‣ 7 of 17 orders has obligate-cave species in terrestrial and freshwater environments. Brazil and China has troglobic in Amphipoda, Bathynelllacea, Isopoda and Decapoda. Brazil also has in Spelaeogriphacea. 346 obligate subterranean species in North America, 68 in China [2], 104 in Mexico [5] and 147 in Brazil.
BATHYNELLACEAE ‣ 253 spp. are troglobic worldwide: 106 in Europa [30], 27 in Africa [7], 15 in Russia and adjacent countries [21], 1 in China [2], 83 in Australia [14], (2:7/)23 in USA [33 | mainly California and Texas], 1 in Venezuela [17 | Parabathynellidae], and 1 in Brazil, a undescribed species collected in Mato Grosso do Sul state [MS/1]. Troglobic Bathynellacea absent in Mexico.


ANASPIDACEAE ‣ five families from Australia, New Zealand and southern South America. All members in Psammaspididae (2, Australia), Stygocarididae (8, Australia 1, New Zealand 2, Chile 2, Argentina 3) and Patagonspididae (1, Argentina) are stygobitic, just like three Anaspididae from S Australia.
AMPHIPODA ‣ 997 troglobic worldwide documented in this article: 438 in Europe [30], 69 in Africa [7], 41 in Russia and adjacent countries [21], 24 in Iran [8 | 9 | 10], (5/)26 in China [2], 3 in Korea (SEE), 3 in SE Asia to New Guinea [22], 144 in Australia [12], (11:24/)192 in USA/Canada [33 | ], (2:)22 in Mexico [5 | Bogidiellidae and Hadziidae], 3 in Cuba [13], 4 in Jamaica [24], 3 in smaller islands of Caribbean [23], (2:)2 in Venezuela [17 | Bogidiellidae and Hyalellidae], and (4:5/)23 in Brazil: Hyalella (5), Megagidiella (2), Potiberaba (5, VIDEO), Spelaeogammarus (10) and Seborgia (1). Five undescribed species in Brazil: 4 collected in Rio Grande do Norte [RN/1] and 1 in Mato Grosso do Sul [MS/1] states. USA has (11:24/)192 troglobic Amphipoda, in Aoridae (1/1, Hawaii), Artesiidae (1/2, Texas), Brevitalitridae (1/2, Hawaii), Crangonyctidae (5/165, 139 in Stygobromus), Eusiridae (1/3, Hawaii), Gammaridae (1/3, Illinois, Pensylvania, Virginia and West Virginia), Hadziidae (8/8, Hawaii and Texas), Hyalellidae (1/1, Nevada), Melitidae (2/3, Hawaii), Parabogidiellidae (2/2, Texas), and Seborgiidae (1/2, Texas), by [33].
ARTESIIDAE










BOGIDIELLIDAE


HYALELLIDAE





MESOGAMMARIDAE






SEBORGIDAE


THERMOSBAENACEA ‣ (4:8/)45 spp. worldwide (Catalogue of Life | Brazilian Metazoa), one of the most enigmatic and challenging for determining troglobic status. Many of the 45 known species inhabit wells, cave lakes, mines, cenotes, interstitial environments, cold and hot springs, and aquifers with salinity ranging from oligohaline to polyhaline, as well as marine and anchialine caves in various regions of the world (H.P. Wagner, Monography, 1994, and recent described species). A careful and detailed analysis allows only ten species to be considered truly troglobic in freshwater environments, in Cuba (1, Tethysbaena vinabayesi), Puerto Rico (1, T. colubrae), USA (2, Tethysbaena texana and Salishbaena kootenai), France (1, T. exigua), Italy (T. argentarii), Thailand (2), Cambodia (1) and W Australia (1, Halosbaena tulki).
SPELAEOGRIPHACEA ‣ a fully freshwater order with (1:3/)4 spp. in Spelaeogriphidae [2], all troglobic, in WC Brazil (Potiicoara), NW Australia (Mangkurtu), and South Africa (Spelaeogriphus). Brazilian member is troglobic Potiicoara brasiliensis Pires, 1987, known only caves from Lagoa Azul in Bonito municipality, Gruta Ricardo Franco near Corumbá municipality, and Gruta do Curupira in the Araras Mountains, Mato Grosso state (Vanin, Zootaxa, 2012).


DECAPODA ‣ four infraorders of Decapoda has troglobic species. Caridea includes a huge of shrimps. Astacidae includes more than 40 stygobitic species of crayfishes of different genera and in environments of temperate and tropical freshwater caves, for Papua New Guinea, Cuba, Mexico, and North America. Anomura includes troglobic (4, in Aegla) only in SE Brazil (and marine forms in Canary Is.). Brachyura has troglobic crabs in Pseudothelphusidae and Trichodactylidae [2].
175 troglobic documented in this article worldwide: 16 in Europe [30], 15 in Africa [7], 7 in Russia and adjacent countries [21], 23 in China [2], 22 in SE Asia to New Guinea [22], 40 in USA/Canada [2], 27 in Mexico [5], 13 in Cuba [13], 2 in Jamaica [24], 1 in Puerto Rico [23], 3 in América Central (Guatemala and Belize, SEE), 2 in Venezuela [17 | Chaceus caecus and Eudaniella sp.], and 4 in Brazil. Mexico has 20 spp. of troglobic freshwater Decapoda, in Alpheidae (shrimp, 1, Potamalphaeops stygicola), Palaemonidae (shrimp, 9), Cambaridae (crayfishes, 5), Pseudothelphusidae (crabs, 4) and Trichodactylidae (crabs, 1). USA has (3:7/)44 spp. [33], in Procarididae (shrimps, 2/2, both in Hawaii), Cambaridae (5/35, crayfishes, mainly in Alabama, Florida and Tennessee), Atyidae (shrimps, 4, Palaemonias in Kentucky and Alabama, and Halocaridina in Hawaii), and Palaemonidae (shrimps, 2/3, Texas and Florida).
AEGLIDAE


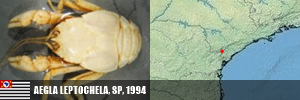

ISOPODA ‣ this order, along with Collembola and Coleoptera, are among the major groups in terms of troglobic diversity worldwide. Our survey documented 880 troglobic species, including 337 in Europe [30], 29 in Russia and adjacent countries [21], 71 in Africa [7], 3 in Iran [8 | 9 | 10], 18 in China [2], 22 in SE Asia to New Guinea [22], 94 in Australia [12], 1 in Vanuatu [22], (11:)52 in Mexico [5], 2 in Guatemala [31], 7 in Cuba [13], 4 in Jamaica [24], 3 in other Caribbean islands [23], 5 in Venezuela [17 | Calabozoidae, Cirolanidae, Sphaeroniscidae, Oniscidae and Pillosciidae], 2 in Colombia [19 | Armadillidium sp. and Diploexochus cacique], 1 in Peru [16], 1 in Bolivia [15], and (12:)136 in Brazil (at least 28 genera), 64 undescribed, in Pará [PA/1][PA/2], Tocantins [TO/1], Rio Grande do Norte [RN/1], Minas Gerais [MG/1][MG/4][MG/6][MG/10], Bahia [BA/1][BA/4][BA/6][BA/7], Espirito Santo [ES/1], São Paulo [SP/2], Paraná [PR/1], Goias [GO/1] and Mato Grosso do Sul [MS/1]. USA has (7:21/)118 troglobic Isopoda, in Asellidae (11/90, 56 in Caecidotea), Cirolanidae (3/4, Texas and Virginia), Ligiidae (1/1, California), Microcerberidae (1/3, Texas), Philosciidae (1/4, Hawaii), Stenasellidae (1/2, Florida and Texas), and Trichoniscidae (3/14), by [33].
CRINOCHETA


ONISCOIDEA


ARMADILLIDAE





BALLONISCIDAE




BRASILEIRINIDAE


CALABOZOIDAE






CIROLANIDAE






DUBIONISCIDAE


PHILOSCIIDAE










PLATHYARTHRIDAE




















PUDEONISCIDAE




SCLEROPACTIDAE












STYLONISCIDAE (VIDEO)

































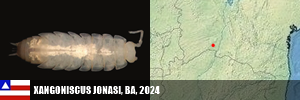































TRACHELYPODIDAE


10.9 BRANCHIOPODA ‣ troglobic forms occur only in Europe, in Spain, Romania, France, Slovenia and Bosnia & Herzegovina, all in Diplostraca [2].
10.10 COLLEMBOLA ‣ a total of 691 troglobic Collembola species were recorded in this study, including 338 in Europe (Fiera, C. et al, JZSER, 2021), (7:16/)100 in the U.S.A and Canada [2], 92 in Brazil, (9:)42 in Mexico [5], 42 in China [2], 35 in Russia and neighboring countries [21], 7 in Cuba [13], 5 in Africa [7], 2 in Jamaica [24], 2 in Peru and Bolivia [15 | 16], 1 in America Central [23], 1 in Argentina (SEE) and 1 in Venezuela [17]. Undoubtedly, thousands of additional species remain undiscovered in caves around the world. All orders of Collembola has troglobic in Brazil (Zeppelini, Pre Print, 2021 | G.A.S. Vaz et al., Acta Oecologica, 2024). Onychiurus acuitlapanensis Palacios-Vargas & Deharveng, 1982 (Onychiuridae) is a unique troglobic Collembola from Venezuela, known also from Guerrero state in Mexico (Galán & Herrera, BSVE, 2006).
UNDEFINED ORDER ‣ a species identified in N Minas Gerais [MG/5] cannot be detected at the order level.

SYMPHYPLEONA ‣ two troglobic species of this order known in SE Bahia [BA/1][BA/6] cannot be detected at the family level. All troglobic members of this order [4] belongs Arrhopalitidae, Bourletiellidae and Sminthuridae, all present in Brazilian caves. Mexico has 4 troglobic spp. in two families. USA has 31 spp., all Pygmarrhopalites (Arrhopalitidae), mainly in Virginia.


ARRHOPALITIDAE ‣ 16 troglobic in Brazil, especially diversified in temperate regions of northern Hemisphere, also present in South America and SE Asia, but absent in Africa. 8 undescribed troglobic species in Brazil, in Rio Grande do Norte state [RN/1], Bahia [BA/2][BA/4], and Minas Gerais [MG/3][MG/10] states.
















BOURLETIELLIDAE ‣ One undescribed troglobic species, in Pará state [PA/2].


SMINTHURIDAE ‣ cave species widely in southen Hemisphere, with Adelphoderia in Australia, Troglospinotheca in Argentina, Troglobentosminthurus and Pararrhopalites in the tropics, all poorly known taxonomically. 4 undescribed troglobic species, in Bahia [BA/1] and in Minas Gerais [MG/3] states.










PODUROMORPHA ‣ troglobic Poduromorpha belong in four of the nine families of Poduromorpha order: Gulgastruridae, Hypogastruridae (unique with troglobic in Brazil), Onychiuridae, and Neanuridae. Troglobic Hypogastruridae of oligotrophic habitats are often characterized by slender or extremely slender claw, but elongation of appendages is moderate; guanobitics have usually reduced eyes and pigment and sometimes elongated claw like several Pseudacherontides. Three undescribed troglobic species in Brazil, in Bahia [BA/7] and Minas Gerais state [MG/2][MG/10]. Mexico has 11 troglobic species in order, in all four 4 families. USA has (3:7/)20 spp. in Hypogastruridae (3/10, mainly Colorado and Alabama), Neanuridae (3/3, Hawaii and Texas), and Onychiuridae (1/7, widely distributed), by [33].
PODUROMORPHA UNDETERMINED FAMILY


HYPOGASTUTIDAE




ENTOMOBRYOMORPHA ‣ order is constituted by nine extant families, six of them include troglobic and 4 has of them in Brazil (35 undescribed species). Four species of this order known in Pará [PA/2], SE Bahia [BA/1][BA/7] and one in São Paulo [SP/3] cannot be detected at the family level. Mexico has 27 spp. in 4 families. USA has (3:8/)50 spp. in Entomobryidae (5/40), Oncopoduridae (1/7), and Tomoceridae (2/3), by [33].
UNPLACED AT FAMILY LEVEL




CYPHODERIDAE ‣ are all phyletically blind and unpigmented; most are myrmecophilous or termitophilous, but Cyphoderus and Troglobius have troglobic-guanobitic species in tropical caves. None exhibits appendage or claw elongation. 17 troglobic spp. in Brazil, one described and 16 undescribed, in Pará [PA/2], Rio Grande do Norte [RN/1], Minas Gerais [MG/1], São Paulo [SP/2], Mato Grosso do Sul [MS/1] and Goiás [GO/1] states.


















ENTOMOBRYIDAE ‣ subterranean radiations are known for many genera, in various regions of the world. Nine troglobic species undescribed in Brazil, in Rio Grande do Norte [RN/1], Bahia [BA/3], Minas Gerais [MG/3] and Mato Grosso do Sul [MS/1] states.




















ISOTOMIDAE ‣ five undescribed species in Brazil, 4 from Pará [PA/2] and one from São Paulo state [SP/2].






PARONELLIDAE ‣ this family is the most diversified Collembola in many tropical caves but are absent in large areas like southern Sunda Islands. 15 undescribed troglobic species in Brazil found in Bahia [BA/3][BA/6][BA/6], Minas Gerais [MG/3][MG/5][MG/10], Goiás [GO/1], Mato Grosso do Sul [MS/1], and São Paulo [SP/2] states.




























NEELIPLEONA ‣ 15 troglobic described species, all in Europe and Asia, and at least one undescribed, known from SE Bahia state, E Brazil [BA/6].
UNPLACED AT FAMILY LEVEL


10.11 DIPLURA ‣ following Sendra et al. (Zoological Journal of Linnean Society, 2021) and complementar sources, 165 described species are troglobic in Europe and adjacent islands except Russia and adjacent countries (110), Russia and adjacent countries (8), Magreb (3), South Africa (1), Lebanon (1), Turkmenistan (1), India (1), Myanmar (1), Indonesia (1), Papua New Guinea (2), China (5), Japan (1), Australia (1), Mexico (8, two Litocampa, two Juxtlacampa, one Oncinocampa and three Tachycampa), Guatemala (1, Juxtlacampa), and one described species in Brazil — Oncinocampa trajanoae Condé, 1997, from the Alto do Ribeira karst, São Paulo state [6]. USA has (2:4/)10 spp. (Condeicampa, Mixojapyx and Occasjapyx one each, and 7 Litocampa) in Campodeidae and Japygidae, by [33].
Additionally, nine undescribed troglobic species have been collected in in Brazil, in Rio Grande do Norte [RN/1], Minas Gerais [MG/1][MG/2] and Bahia [BA/2][BA/3][BA/5] states.
UNDETERMINED FAMILY


CAMPODEIDAE


JAPYGIDAE



PROJAPYGIDAE






10.12 INSECTA ‣ members of 13 orders of insects occur as cave-dwellers, 12 with troglobic [4]. 290 troglobic insects occur in North America: Coleoptera (269), Dermaptera (1), Diptera (1), Hemiptera (9), Orthoptera (3), and Zygentoma (7). 202 in China [2]. Troglobic Insecta in Mexico are 65 spp. in 4 orders: Zygentoma (13), Orthoptera (4), Hemiptera (3, all Homoptera) and Coleoptera (43). Brazil has 86 spp. in eight orders: Zygentoma (7), Orthoptera (5), Hemiptera (12), Coleoptera (50), Blattodea (8), Diptera (1), Dermaptera (1) and Hymenoptera (2).
EPHEMEROPTERA ‣ a troglobic species within this order has been reported from Colombia [19], and it is provisionally accepted here. However, its validity still requires thorough confirmation in a peer-reviewed publication. If confirmed, it would represent the first described species of its group worldwide.
NOTOPTERA ‣ order formed by Manthophasmathodea and Grylloblatoidea, the former wihtout mentions about cave-dwellers. In latter, mainly species are epigean, but some species of Galloisiana and Namkungia in South Korean inhabit montane sites and many low elevation caves, wuth several reduced or eyeless species appear to be limited to cave environments. Some Grylloblatta populations are restricted to caves, morphological adaptations to the cave environment (troglomorphy) are not known, with the exception that cave-dwelling species lack pigmentation (Wipfler, B. et al, Journal of Insect Biodiversity, 2014). Besistes these reports, none troglobic is fully mentioned in literature.
ZYGENTOMA ‣ although there are records of troglobic Zygentoma from Australia, Thailand, New Guinea and the Philippines, they are only mentioned in species checklists for the Balkans, Cuba, and Brazil. 8 spp. in USA (6 Texoreddellia and two Speleonycta, both Nicoletiidae), (3/)13 in Mexico, 1 in Guatemala, 3 in Cuba, and 7 in Brazil, one described and six undescribed in Bahia [BA/2][BA/3][BA/4], Minas Gerais [MG/1], and Espírito Santo [ES/1] states, including two in Lepismatidae. When published, it will be the first oficial troglobic species in this family.
UNDETERMINED FAMILY


LEPISMATIDAE


NICOLETIIDAE




ORTHOPTERA ‣ 63 troglobic Orthoptera are documented: 1 in Balkans [29], 1 in Africa [7], 40 in China [2], 1 in SE Asia [22], six in Hawaii [33 | all Gryllidae], 6 in Mexico [5 | all Phalangospsidae], 4 in Cuba [13], 2 in Venezuela [17] and six in Brazil — all in Phalangospsidae, including one undescribed in Bahia [BA/4] state. All cave species of Orthoptera are members of Ensifera, which are omnivorous or scavengers, and none of Caelifera, which are phytophagous. Most belong to the large families Gryllidae, Phalangopsidae, Trigonidiidae, and Rhaphidophoridae. Rhaphidiophoridae from Venezuela are the only aquatic or semi-aquatic troglobic Orthoptera (Galán & Herrera, BSVE, 2006). Some troglophylic Phalangopsis (Phalangopsidae, Junta et al., Zootaxa, 2020) occur in Amazon region.
PHALANGOSPSIDAE





COLEOPTERA ‣ approx. 2,500 spp. are troglobic worldwide are cited in [4]. Three families, Carabidae, Staphylinidae (including Pselaphinae and Scydmaeninae), and Leiodidae count for 98% of the diversity of troglobic and troglophilic beetles, with 1,180, 110, and 599 spp., respectively [4].
There is a considerable amount of scattered literature on Coleoptera. [4] discusses higher-level groupings, while Hlavac et al (Subterranean Biology, 2006) provides a species list for Staphylinidae. In most parts of the world, Coleoptera diversity is relatively well documented. The main exceptions are Europe, Middle East, Indian Subcontinent, NE Asia, Australia, Pacific, America Central, Caribbean, and South America from Ecuador to Argentina. Our current numbers remain quite limited, with only 1,529 species recorded — 384 of them from Western Europe in Balkans [31], 25 in Spain (Staphylinidae non Pselaphinae, SEE), 54 in Ariege in France (SEE), 143 in Romania (SEE), 1 in Italy (Staphylinidae non Pselaphinae, SEE), 23 in Africa [7], 36 in Russia and adjacent countries [21], 159 in China [2], 1 in Korea (Staphylinidae non Pselaphinae, SEE), 82 in Japan (SEE | SEE), 18 in SE Asia [22], 184 in Australia [12], 44 in Mexico [5 | Carabidae-30, Dytiscidae-1, Histeridae-4, Leiodidae-8, Ptinidae-1], 6 in Cuba [13], 4 in Jamaica [24], 1 in Guatemla [23], 3 in Venezuela [17 | Carabidae-1, Dysticidae-1, Catopidae-1], 5 in Colombia [19 | Staphylinidae-2, Carabidae-1, Nitidulidae-1, Passalidae-1], 1 in Ecuador (SEE), 3 in Bolivia [15] and 88 in Brazil. USA has (9:31/)284 spp. of troglobic Coleoptera, in Anobiidae (1/1, California), Carabidae (13/193), Curculionidae (2/2, Texas), Dryopidae (2/3, Oregon and Texas), Dytiscidae (3/3, Texas), Elmidae (1/4, Texas), Leiodidae (2/22, mainly Alabama and Tennessee), Staphylinidae (7/54, mainly in Alabama), and Tenebrionidae (2/2, California and Texas), by [33].
(6:)88 troglobic in Brazil, being 64 undescribed in Pará [PA/2], Bahia [BA/1][BA/2][BA/4][BA/5][BA/6][BA/7], Mato Grosso do Sul [MS/1][BR/1], Goiás [GO/1], Minas Gerais [MG/1][MG/2][MG/6][MG/10], Rio Grande do Norte [RN/1], São Paulo [SP/2], Tocantins [TO/1] states, in 7 families. Truly troglobic species at Scarabeoidea are yet to be found in Brazil (C.M.A. Correa et al., Revista Brasileira de Entomologia, 2022).
CARABIDAE











































DYTISCIDAE
















EUCNEMIDAE


HISTERIDAE


HYDROSCAPHIDAE

STAPHYLINIDAE




























BLATTODEA ‣ 26 troglobic Blattodea documented in this work, mainly saprophagous and from Old World: 5 in Africa [7], 8 in SE Asia [22], 1 in Cuba [13], 1 in Jamaica [24], 1 in Venezuela [17 | Paranocticola venezuelana Bonfils, 1987], 1 in Colombia [19], 1 in Ecuador (Ischnoptera peckorum Roth, 1988 from Galapagos Is., Ectobiidae, SEE), and 8 in Brazil: Litoblatta camargoi Gutiérrez, 2005 (Blattellidae), from Bahia state (Guterrez, Solenodon, 2005), and additional 7 undescribed troglobic species, in Bahia [BA/1][BA/2][BA/3][BA/6][BA/7] and Minas Gerais [MG/2][MG/6] states.








PSOCOPTERA ‣ besides data in [4] for several possible troglobic in New World, the only clearly troglobic representative of this order in New World is the prionoglaridid Speleopsocus chimanta Lienhard, 2010 known from a humid cave in SE Venezuela (C. Lienhard & R. L. Ferreira, Revue suisse de Zoologie, 2015).
DERMAPTERA ‣ there are only four troglobic in this order: Anataelia troglobia MartÌn & OromÌ, 1988 in Canary Is., Spain, Anisolabis howarthi Brindle, 1980 in Hawaii [33], Mesodiplatys falcifer Kamimura & Ferreira, 2018 (Diplatyidae) from Carinhanha municipality in Bahia state in Brazil, and one undescribed from Colombia [19].


HEMIPTERA ‣ 33 documented troglobic species worldwide: 5 in Spain (SEE | SEE), 1 in Africa [7], 1 in China [2], (3:4/)10 in Hawaii [33], 3 in Jamaica [24], 1 in Colombia [19], 1 in Argentina (SEE) and 13 in Brazil, in six families in all three suborders with cave members. All three Mexican troglobic of this order belongs Fulguromorpha, and all 10 troglobic in USA are endemic to Hawaii, in Cixiidae (2/8, Fulguromorpha), Mesoveliidae and Reduviidae (2/2, both Heteroptera). One troglobic species in Cydnidae was collected in Colombia [19]. The five undescribed troglobic in Brazil was collected in Pará [PA/2], Bahia [BA/4][BA/5], and Mato Grosso do Sul [MS/1].
AUCHENORRHYNCHA/CIXIIDAE
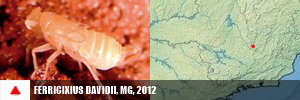



AUCHENORRHYNCHA/DELPHACIDAE


AUCHENORRHYNCHA/KINNARIIDAE (VIDEO)


HETEROPTERA/DIPSOCORIDAE


HETEROPTERA/HYDROMETRIDAE


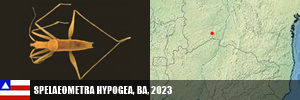

STENORRHYNCHA/ORTHEZIIDAE


TRICHOPTERA ‣ a troglobic species within this order has been reported from Colombia [19], and it is provisionally accepted here. However, its validity still requires thorough confirmation in a peer-reviewed publication. If confirmed, it would represent the first described species of its group worldwide.
DIPTERA ‣ the discussion about troglobic diptera is confusing because all troglobic members of this order are not or weakly modified for cave life. Several non-hematophagous Diptera are troglobic: 2 in Balkans [29], 3 in Africa [7], 2 in China [2], 2 in SE Asia [22], (3:3/)3 in USA [33 | Spelobia tenebrarum Aldrich, 1897 in 13 states, and two in Hawaii], and 2 in Colombia [19 | Drosophilidae and Mycetoohilidae one each]. The only hematophagous species firmly established as a troglobic, Deanemyia maruaga Alves, Freitas & Barrett, 2008 from N Brazil (known only from Maruaga cave, in Presidente Figueiredo, Amazonas state), is parthenogenetic, nonvector, and can complete its full life cycle in cave [4]. More details in Veracilda Ribeiro Alves et al. (Mem. Inst. Oswaldo Cruz, 2011); this species is corroborated by [6].


LEPIDOPTERA ‣ three described troglobic species are known in this order. Tinea microphthalma Robinson, 1980 (reduced-eyes Tineidae, Philippines) and Schrankia howarthi D. Davis & Medeiros, 2009 (Erebidae, Hawaii, SEE), which presents several regressive traits: flightlessness, reduced pigmentation, and reduced eye size [4 | 33]. A third troglobic species was collected in Colombia (Angarita-Sierra, CCE/2018). Troglobic species in this order are completely unknown in Brazil.
HYMENOPTERA ‣ three described troglobic are known in this order, all ants: Leptogenys khammouanensis Roncin & Deharveng, 2003, collected in oligotrophic habitats of a Laotian cave [4]; Aphaenogaster gamagumayaa Naka & Maruyama, 2018, from Okinawa, Japan (Naka & Maruyama, Zootaxa, 2018); and Yavnella laventa Griebenow, Moradmand & Isaia, known from Fars province, SW Iran (Z. H. Griebenow et al., Invertebrate Systematics, 2022). Three undescribed troglobic was collected in Pará[PA/2], Bahia [BA/1] and Tocantins [TO/1] states in Brazil.




11.1 PHYLUM CRANIATA/ACTINOPTERI ‣ Subterranean Fishes of the World (in December 08, 2024) lists 314 spp. of troglobic Actinopteri among 11 orders. In this source Brazil appears with 44 spp., in second diversity worldwide, after China (107, 105 in Cypriniformes, mainly in Yunnan region), and ahead Mexico (20) and USA (22). The four countries together have 193 spp. (USA and Mexico shared one spp.), more than half of the world amount of species.
Although Poecilia mexicana Steindachner, 1863 (Poeciliidae, Cyprinodontiformes) and Astyanax aeneus Günther, 1860 (Characidae, Cypriniformes) are listed in the SFW, we reject its troglobic status here, as it is a species with epigean populations (Wikipedia) for both and is therefore excluded from the scope of this article. The figures below already reflect this adjustment.
OUR LIST FOR BRAZIL
Of the 45 troglobic fish species cited for Brazil in SFW (44 nominally + Phreatobius undescribed species Aripuana), six are excluded, and the remaining 39 are added to one undescribed in Bahia [BA/4], totaling 40 species accepted in this article, in Characiformes (1:1/1), Gymnotiformes (1:1/1) and Siluriformes (4:10/38). The six species rejected here are Isbrueckerichthys alipionis Derijst 1996 (Loricariidae, isolated cave population of an otherwise epigean species, SEE), and five undescribed Phreatobius (Heptapteridae, being four in Rio Negro basin and one in Aripuanã river basin — full details in Cunha, Thesis, 2018).
All Brazilian troglobic fishes are quite small, with only three species exceeding 100 mm. A manual search for size data revealed: Pimelodella kronei (20.2 cm, PlanetCatfish), Rhamdia enfurnada (147 mm, SEE), and Eigenmannia vicentespelaea (up to 123 mm, SEE). P. kronei holds two remarkable distinctions: it is the oldest described troglobic species in Brazil (1907) and, based on the sizes listed above — and assuming no Brazilian troglobic invertebrate exceeds 100 mm — it is also the largest troglobite in the country.
ORDERS IN OLD WORLD
Two orders has troglobic only in Old World: Anabanthiformes (only in India), and Cypriniformes (Germany, Africa, and Iraq and Turkmenisthan to Malaysia and China).
ORDERS IN OLD AND NEW WORLD
Four orders has troglobic in New and Old World: Siluriformes (Asia, Africa and New World), Ophidiformes (Caribbean, Mexico, Galapagos and Indonesia), Gobiiformes (Japan, Madagascar, Australia, Guam, Mexico, Indonesia, Philippines and Papua New Guinea), and Synbrachiformes (India, Australia, Cameroon, Mexico and Venezuela).
ORDERS IN NEW WORLD
Four orders has species only in New World: Characiformes (Mexico and Brazil), Gymnothiformes (Brazil), Percopsiformes (USA) and Perciformes (USA).
DIVERSITY BY COUNTRIES
33 countries in New World has troglobic Actinopteri [14], mainly in tropical New World and tropical Asia, highly diverse in China, Brazil, USA, India and Mexico.
AFRICA (5): Cameroon (1:1/1), Namibia (1:1/1), Somalia (2:3/3), D.R. Congo (1:1/1) and Madagascar (2:2/5).
EUROPE (2): Croatia and Germany, both with a single species.
MIDDLE EAST/CENTRAL ASIA (4): Turkmenistan (1:1/1), Iraq (2:3/3), Oman (1:1/3) and Iran (2:2/5).
E, S & SE ASIA (9): India (7:10/21), Myanmar (1:1/1), Thailand (4:5/9), Laos (2:3/3), Vietnan (3:6/7), Malaysia (1:1/1), China (5:16/107), Japan (1:1/5), Philippines (2:2/3) and Indonesia (3:3/3).
OCEANIA (3): Papua New Guinea (1:1/2), Australia (2:2/3) and Guam (1:1/1).
NEW WORLD (12): USA (3:7/22), Mexico (8:8/20), Belize (1:1/1), Bahamas (1:1/2), Cuba (1:1/9), Venezuela (4:4/5), Colombia (1:1/10), Ecuador (1:1/2), Peru (1:1/1), Bolivia (2:2/2), Argentina (1:1/1) and Brazil (7:14/44). Mexico has (7:7/)19 spp. in four orders, includng Rhamdia (11, Heptapteridae), Astynax (1, Characidae), Prietella (2, Ictaluridae), Lucifuga (1, Bithytidae), Typhlias (1, Dinematichthyidae), Caecieleotris (1, Eleotridae) and Ophisternon (1, Synbranchidae). In the USA there are (3:7/)22 spp. in 3 orders, in genera Prietella, Satan, Trogloglanis (Ictaluridae, 3), Amblyopsis, Speoplatyrhinus, Troglichthys (Ambryopsidae, 16) and Cottus (Cottidae, 3).
All genera with troglobic fish in South America have them in Brazil except Astroblepus (Ecuador and Peru one troglobic each, Astroblepidae), Chaetostoma (1 troglobic, in Ecuador, Loricariidae), Silvinichthys (1 troglobic, in Argentina, Trichomycteridae), Ogilbia (1 troglobitc, Galapagos, Dinematichthyidae) and Synbranchus (1 troglobic, Venezuela), all of them with troglobic only on the continent.
ENDEMIC GENERA AND SHARED SPECIES
In New World, exclusively underground endemic genera are Trogloglanis and Satan, both monotypic of Ictaluridae endemic to the USA, Amblyopsis, Triglichthys and Speoplatyrhynus, all of Amblyopsidae, which is, as a whole, an endemic family of the U.S.A; and Stygichthys (Characidae), endemic to SE Brazil. No other country has entirely underground endemic genera of fish. Only Prietella phreatophila Carranza, 1954 (Mexico/USA) and Phreatobius sanguijuela (Brazil/Bolivia) are not national endemisms. Altogether there are 95 spp. in 12 countries, distributed in 30 genera of 16 families in 9 orders.
RECORDS WORLDWIDE
Neolissochilus pnar Dahanukar, Sundar, Rangad, Proudlove & Raghavan, 2023 (Cyprinidae) endemic to NE India is the largest troglobtic fish worldwide, possibly the largest of all troglobic (Novotaxa).
TAXONOMY
CHARACIFORMES ‣ troglobic in this order includes only two Characidae: Astyanax jordani Hubbs & Innes, 1936, from San Luiz Potosi and Tamaulipas in Mexico, and Stygichthys typhlops Brittan & Böhlke, 1965, from N Minas Gerais state, E Brazil.


GYMNOTIFORMES ‣ the Brazilian cave Eigenmannia vicentespelaea Triques, 1996 (Sternopygidae) is the unique stygobitic in this order worldwide, known only from NE Goias state.


SILURIFORMES ‣ speciose order with 82 cave species worldwide in 11 families, four of these has troglobic members in Brazil.
CALLYCHTHYIDAE


HEPTAPTERIDAE


















LORICARIIDAE


TRYCHOMYCTERIDAE


















11.2 PHYLUM CRANIATA/AMPHIBIA ‣ by [2] and [33], 13 amphibians worldwide are obligatory subterranean species, in three genera: Eurycea (9, Plethodontidae, in Texas, Arkansas, Kansas, Missouri, Oklahoma, Florida and Georgia), Gyrinophilus (3, Plethodontidae, in Alabama, Georgia, Tennessee and West Virginia) and Proteus anguinus Laurenti, 1768 (Proteidae, Dinaric Mountains in NE Italy, Slovenia, Croatia, and Bosnia-Herzegovina), the only stygobitic salamander in Europe and unique member of their family in continent, reaches a length of more than 25 cm, making the second largest troglobic (stygobitic) known anywhere after Neolissochilus pnar (Actinopteri) from India. [5] does not recognize any cave salamander in Mexico.
[2] cites one cave-dwelling Anura in China, however is fully rejected here as troglobic, being excludes in this count. Sperandei, V.F. et al. (Biota Neotropica, 2024) discuss Anura in caves in Brazil, citing 54 species that have already been collected in caves. Oreobates antrum az-Silva, Maciel, Andrade and Amaro, 2018 has only been found inside caves, in the states of Goiás and Tocantins (Motta, A. et al, Herpetology Notes, 2020). However, there are no known records of troglobic Anura worldwide.
LAST UPDATES
October 20, 2025 ‣ addition of 122 undescribed troglobic species (including first record of a troglobic Schizomida in Brazil) collected in various inventories in the Carajás Range, Pará state, compiled by Trevelin et al. (Ecological Indicators, 2019).
October 13, 2025 ‣ addition of nine undescribed species for Pará, originating from Paraíso Cave in Aveiro (SEE).
October 12, 2025 ‣ numerical updates and a complete revision were carried out for the USA (including Hawaii and Alaska) and Canada (Niemiller, M.L. et al., Biodiversity and Conservation, 2025), along with partial updates for South Korea (SEE | SEE), Argentina (1 Diplopoda, SEE), and Mexico (1 Opiliona, SEE).
June 14, 2025 ‣ corrections in Actinopteri and inclusion of Endecous spelaeus (Orthoptera, BA, SEE).
May 29, 2025 ‣ addition of Pseudochthonius maquinensis, P. urubuquaqua (ZOOTAXA, 2025), Spelaeogammarus rafaelae, S. lundi (Taxonomy, 2025), Circoniscus paradisus (Nauplius, 2025), and Brasileirinho sergipanus (Zootaxa, 2025).
March 22, 2025 ‣ addition of 20 undescribed species of Padre Cave, Santana municipality, Bahia (SEE), and 13 undescribed species for Paraná state (SEE).
March 16, 2025 ‣ addition of one Hydroscaphidae, collected in Jardim municipality, Mato Grosso do Sul (SEE).
January 05, 2025 ‣ addition of Girardia patiensis and G. alba (BA, Platyhelminthes, Zoologischer Anzeiger, 2025).
December 26, 2024 ‣ addition of 7 spp. of Xangoniscus (Isopoda) from Bahia: X. paiabare, X. tymaopeba, X. ykanhema, X. puku (SEE), Xangoniscus antiquus, X. chaimowiczi, X. jonasi (SEE), subsequent updates, and upadates and correction among Bahian municipalities; addition of two Gastropoda from Carinhanha, Bahia (SEE), Cryptops didi from Iporanda, São Paulo state (SEE); Pseudochthonius limettioides for Caetá, Mariana and Santa Barbara in Minas Gerais (SEE), P. aware for Santa Maria Vitória in Bahia (SEE), and Paleotoca diminas for Presidente Prudente in Minas Gerais (SEE), and addition of 13 undescribed species after (G.A.S. Vaz et al, Acta Oecologica, 2024), knwon from Pedro Cassiano cave, Carinhanha, Bahia state.
December 15, 2024 ‣ update and revision, text and terminology standardization, HTML code correction, topic structuring, language smoothing, species authorship, visual optimization, and information accuracy throughout the post.
November 13, 2024 ‣ corrections and simplification of the writing, code optimization, reordering of data about Indonesia, Australia, inclusion of data from Africa and Croatia.
April 23, 2024 ‣ exclusion of Endecous apterus and E. peruassuensis (Orthoptera), rejected in Carvalho, P.H.M. et al. (Zootaxa, 2023).
25∙04∙2024 ‣ addition of 11 new described species: Pinelema elinae (BA), Loxosceles boqueirao (BA), L. bodoquena (MS), Spaeleoleptes gimli (BA), Cayenniola albaserrata (BA), Trichorhina baiana (BA), Ctenorillo iuiuensis (BA), Circoniscus mendesi (PA), Circoniscus xikrin (PA), Kadiweuoniscus rebellis (MS) and Endecous vitreus.
22∙01∙2024 ‣ inclusion of 10 new undescribed species in Aguas Claras System, Carinhanha (BR), in Araneae, Coleoptera, Diplura, Hemiptera and Dilplopoda (SEE).
22∙01∙2024 ‣ exclusion of Pseudonannolene canastra (Diplopoda), synonimized under P. ambuatinga, with ratification of accounting on several topics and readaptation of the P. ambuatinga map (SEE).
20∙10∙2023 ‣ inclusion of Caraiboscia jabutiensis (Isopoa, MT, SEE), Gabunillo enfurnado, Venezillo moreirai, V. limai (Isopoda, BA, SEE) and Spelaeometra hypogea (Hemiptera, BA, SEE).
16∙10∙2023 ‣ of records of undescribed troglobic species in three regions: Lapa Nova, Vazante, MG (6 spp., SEE), Lagoa Santa, MG (4 spp., SEE) and Presidente Olegário, MG (3, SEE).
13∙10∙2023 ‣ inclusion of 5 new Pectenoniscus (Isopoda, SEE) and Pseudochthonius lubueno (SEE).
10∙09∙2023 ‣ updates in undescribed species in Bahia state, with a inclusion f 30 news undescribed (Gallão et al., Diversity, 2023); inclusion of Plutogeophilus jurupariquibaba, former undescribed (Chilopoda, [SP/2], SEE); inclusion of Benthana alba, Benthanoides amazonicus, B. tarzan (Cardoso & Ferreira, Zootaxa, 2023), Ferricixius michaeli and F. goliathi (Ferreira et al., Zootaxa, 2023).
25∙07∙2023 ‣ updates in troglobic Hirundina and Polychaeta at Annelida.
20∙07∙2023 ‣ updates in Amblypygi, with a remarkable new record in Colombia.
30∙06∙2023 ‣ updates about troglobic sponges, and troglobits in Mexico, and inclusion of Phretobius sanguijuela, with general updates in Heptapteridae.
19∙06∙2023 ‣ inclusion of Endecous infernalis, former undescribed [BA/1](SEE).
09∙06∙2023 ‣ add three new species: Pseudochthonius koinopoliteia, former undescribed [BA/1], P. diamachi, P. pali (Prado & Ferreira, Zootaxa, 2023).
03∙01∙2023 ‣ updates in Collembola, inclusion of Iandumoema cuca, I. gollum, I. stygi (Opiliones), Dobrodesmus mirabilis (Dobrodesmidae), Ardistomis ferreirai (Carabidae), Metopioxys carajas, Oxarthrius aurora, O. inexpectatus (Staphylinidae),Leptus sidorchukea (Erythraeidae), Tonton itabirito, T. matodentro (Microstigmatidae), Pararrhopalites sideroicus (Sminthuridae), Onciurosoma troglobium (Paradoxosomatidae), Loxocelis troglobia (Sicariidae), Brasilomma enigmatica, Indiani gaspar, Paracymbiomma bocaina, P. pseudocaesus (Prodidomidae), Ochyrocera dorinha, O. rosinha, O. ungoliant, Speocera babau, S. pinima, S. piquira (Ochyroceratidae), Charinus cearensis, C. diamantinus, C. euclidesi, C. puri, C. renneri (Charinidae), Aegla charon (Aeglidae), Lavajatus moroi (Subulinidae), and exclusion of Troglorhopalurus lacrau (Scorpiona), Isoctenus corymbus (Ctenidae), Lygromma ybyguara (Prodidomidae) and Spiripockia umbraticola (Pomatiopsidae), by indications in [6], and inclusion of Eukoenenia mocororo.
08∙08∙2022 ‣ conclusion of a huge revision of all data from this page, inc. several notes for Mexico and USA
03∙07∙2022 ‣ a huge revision, including c. 280 new species in list, mainly undescribed.
12∙06∙2022 ‣ inclusion of Perigona spelunca (Carabidae).
26∙11∙2021 ‣ inclusion in the list of troglobic in Brazil of 6 spp. de Matta (Araneae) and updated the text to include this addition.