LAST UPDATE IN FEB 17, 2026
CHECKLIST OF PHYLLA
1 ABSTRACT
We present here a detailed and taxonomically stable analysis of the diversity of Metazoa in Brazil and worldwide. Our numbers indicate 1,596,517 species worldwide in 182,551 genera in 8,139 families, with 127,776 species in Brazil, in 27,509 genera within 3,499 families, as of February 17, 2026. We classify Metazoa into 145 lineages, 123 of which have representatives in Brazil, while 22 are unknown. Groups traditionally recognized as taxonomic units, such as Polychaeta, Crustacea, Reptilia, and Fishes, are analyzed in light of phylogeny, with potential fragmentation. We highlight endemic families and compare Brazilian diversity with that of Mexico, the main competing country in biodiversity in America Latina. Finally, we list some of the most important sources of publication in zoological taxonomy, both in Brazil and globally.
NOMENCLATURE, DEFINITIONS AND CONVENTIONS
We use the notation (z : y /) x, which refers to x species in y genera of z families. For the phylogenetic trees presented here, ![]() represents a clade present in Brazil, and
represents a clade present in Brazil, and ![]() represents a clade not recorded in the country. For the sake of clarity and conciseness, totals for some higher-level taxa are obtained by summing more specific groups, which are properly referenced. References marked as Eschmeyer’s CF/20260217 indicate a citation to the Eschmeyer’s Catalog of Fishes website as consulted on 17 February 2026, with the numerical code specifying the access date.
represents a clade not recorded in the country. For the sake of clarity and conciseness, totals for some higher-level taxa are obtained by summing more specific groups, which are properly referenced. References marked as Eschmeyer’s CF/20260217 indicate a citation to the Eschmeyer’s Catalog of Fishes website as consulted on 17 February 2026, with the numerical code specifying the access date.
DISCLAIMER AND ALERT
In this study, the aim was exclusively to list accepted living species, genera, and families, using the Catalogue of Life as the primary reference, although undescribed species are included for many taxa throughout the text; notably, the recognition of at least two canonical lineages from Brazil stems from this addition. Owing to the fragmented nature of the information available across the different platforms consulted, the need for manual counts, and the inherent possibility of human error associated with this process, the numbers presented should be regarded as specific to this survey rather than as real or definitive values, since a substantial amount of diversity remains to be discovered. The very process of data collection, integration, and systematization involves methodological limitations that may result in internal inconsistencies or inaccuracies.
2 INDEX
SYNOPSIS OF BRAZILIAN ANIMAL DIVERSITY
NATIONAL ENDEMIC FAMILIES
MEGAFAUNA and PALEONTOLOGY
BRAZILIAN CAVE FAUNA
SOUTH AMERICAN BIRDS
3 MAIN REFERENCES
Our work here searched hundreds of bibliographic and digital references, such as websites and platforms, in order to elaborate the most accurate database possible summarizing all the diversity of the Metazoa Kingdom and the level of the main groups within each phylum, and to obtain precise numbers of families, genera, and species in Brazil and worldwide for each group. However, six references deserve special mention due to their extreme relevance in the construction of this text.
▪ Catalogue of Life, a primary reference widely adopted throughout our text and applied to multiple groups for assessing total diversity. For an accurate assessment of the extant diversity of taxa, searches must be conducted using the parameters: [1] accepted and [2] extinct: unknown.
▪ Freshwater Animal Diversity Assessment (Hydrobiologia, 2008) — Balian (2008).
▪ Catálogo Taxonômico da Fauna do Brasil (CTFB), cited here as CFTB, extremely important for several numbers, especially in Insecta, Diplopoda, Maxillopoda, Malacostraca, Arachnida and Platyhelminthes.
▪ Keys to Nearctic Fauna (Thorp and Covich's Freshwater Invertebrates: Keys to Nearctic Fauna, 2016) — KNF/2016.
▪ Keys to Neotropical and Antarctic Fauna (Thorp and Covich's Freshwater Invertebrates: Volume 5: Keys to Neotropical and Antarctic Fauna, 2020) — KNAF/2020.
▪ Copepedia/Animalia
▪ Intreasures (SEE), which provides an important amount of information about endemism by country.
4 LINEAGES and NUMBERS
Zhi-Qiang (Zootaxa, 2013) recognizes 39 extant phyla (SEE), with Myxozoa independent of Cnidaria and Sipuncula and Echiura apart from Annelida; here, we follow Wikipedia and treat Myxozoa within Cnidaria, Xenoturbellida and Acoelomorpha united under Xenoacoelomorpha, Rotifera and Acanthocephala united as Syndermata (Laumer CE et al., Proc. R. Soc. B, 2019 | Giribet et al., BOOK, 2023), and Sipuncula and Echiura within Annelida, based on recent works on the phylum (see text), resulting in 34 phyla: Ctenophora, Porifera, Placozoa, Cnidaria, Xenacoelomorpha, Chaetognatha, Gnathostomulida, Micrognathozoa, Syndermata, Orthonectida, Dicyemida, Gastrotricha, Platyhelminthes, Entoprocta, Cycliophora, Nemertea, Mollusca, Annelida, Brachiopoda, Bryozoa, Phoronida, Loricifera, Kinorhyncha, Priapulida, Nematomorpha, Nematoda, Tardigrada, Onychophora, Arthropoda, Hemichordata, Echinodermata, Cephalochordata, Tunicata, and Craniata.
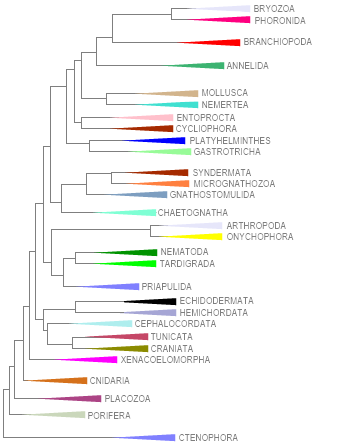
METAZOA PHYLOGENY FOR LAUMER C.E. ET AL. (RSP/2019, 2023), LACKING NEMATOMORPHA, KINORRHYNCHA, LORICIFERA, DICYEMIDA AND ORTHONECTIDA
Brazil presents 31 of the 34 phyla accepted in this work, being one of the richest countries in the world in this regard. The highest worldwide diversities are 32 in the USA, Spain (lacking Orthonectida and Onychophora), Italy, and France (lacking only Micrognathozoa and Onychophora); and 31 in Brazil, Mexico (lacking Micrognathozoa, Orthonectida, and Cycliophora), and Japan (lacking Micrognathozoa, Onychophora, and Cycliophora).
According to our survey, Metazoa comprise 145 canonical lineages (after exclusion of Dorylaimia in Nematoda; requalifications in Chaetognatha, Entoprocta, Phoronida, Bryozoa, Hemichordata, and Placozoa; Thaliacea in Tunicata; and further requalifications in Platyhelminthes, Arthropoda/Pancrustacea, Priapulida, and Cephalochordata). Depending on the phylum, these lineages range from classes to genera (e.g., Priapulomorpha). Of these, 22 — including three entire phyla, the marine parasitic Dicyemida, Orthonectida, and Cycliophora — have never been collected in Brazil, whereas 123 are present. Almost all of these 22 lineages are marine, with records concentrated in North America and Europe, and a few occurring on land or in freshwater (e.g., Polypodizoa and Peripatopsidae).
:: SUMMARY LIST OF ALL METAZOA ORDERS (TEXT)
Salinella salve J. Frenzel, 1892 is a dubious species of a very simple animal that may not exist, but which some have named as the sole member of the phylum Monoblastozoa. It was discovered in 1892 by Johannes Frenzel in the salt pans of Argentina and cultivated in a laboratory by him. This animal has not been found since and its real existence is considered as doubtful (Wikipedia).
5 TABLE
In the table below, in the family/genus/species columns, the first numbers represent the data for Brazil and the second the numbers in world (in parenthesis). Gray lines are the final counting of the phylum. The distribution of the parasites is given according to that of their host.
|
|
MAIN GROUPS |
FAMILIES |
GENERA |
SPECIES |
NOTES and REFERENCES |
|
1. CTENOPHORA |
TENTACULATA |
8 (34) |
10 (53) |
11 (158) |
|
|
NUDA |
1 (1) |
1 (2) |
3 (44) |
|
|
|
|
9 (34) |
11 (48) |
14 (184) |
|
|
|
2. PORIFERA |
CALCAREA |
10 (26) |
23 (96) |
84 (922) |
|
|
HEXACTINELLIDA |
9 (26) |
15 (155) |
22 (750) |
|
|
|
HOLOSCLEROMORPHA |
2 (2) |
4 (12) |
17 (136) |
|
|
|
DEMOSPONGIEAE |
70 (119) |
187 (626) |
539 (8,398) |
|
|
|
|
91 (173) |
229 (889) |
6562 (10,206) |
|
|
|
3. PLACOZOA |
POLYPLACOTOMIA |
- (1) |
- (1) |
- (1) |
Known only from Alassio region, NW Italy. |
|
UNIPLACOTOMIA |
1 (4) |
1 (7) |
1 (22) |
|
|
|
|
1 (5) |
1 (8) |
1 (23) |
|
|
|
4. CNIDARIA |
HEXACORALLIA |
45 (155) |
145 (856) |
227 (4,720) |
|
|
OCTOCORALLIA |
21 (97) |
48 (480) |
76 (4,103) |
|
|
|
CUBOZOA |
4 (8) |
4 (21) |
4 (54) |
|
|
|
HYDROZOA |
79 (157) |
190 (683) |
365 (4,404) |
|
|
|
POLYPODIOZOA |
- (1) |
- (1) |
- (1) |
One sp., fish egg-parasitic from Russia, Iran, Kazakhstan, Moldova, Romania, Canada and USA |
|
|
SCYPHOZOA |
12 (30) |
14 (82) |
20 (320) |
|
|
|
STAUROZOA |
1 (8) |
1 (16) |
2 (55) |
|
|
|
MYXOZOA |
11 (31) |
19 (75) |
221 (3,131) |
|
|
|
|
173 (487) |
416 (2,214) |
915 (16,788) |
|
|
|
5. XENACOELOMORPHA |
XENOTURBELLIDA |
- (1) |
- (1) |
- (6) |
Known only in coasts of SE Japan (1), Sweden (1), Gulf of California in NW Mexico (3) and S California (1). |
|
NEMERTODERMATIDA |
- (2) |
- (6) |
- (18) |
Known only from Swedish west coast, Belgian, E North America, Adriatic, Mediterranean seas, New Guinea, Australia and New Zealand. |
|
|
ACOELA |
9 (16) |
24 (106) |
33 (430) |
|
|
|
|
9 (19) |
24 (113) |
33 (454) |
|
|
|
6. CHAETOGNATHA |
APHRAGMOPHORA |
3 (6) |
12 (23) |
22 (66) |
|
|
PHRAGMOPHORA |
2 (5) |
2 (12) |
3 (68) |
|
|
|
|
5 (11) |
14 (35) |
25 (134) |
|
|
|
7. GNATHOSTOMULIDA |
FILOSPERMOIDA |
- (2) |
- (3) |
- (28) |
Known elsehere from Europe (7), E USA (1), Hawaii (4), Caribbean (4), Fiji (4), Sweden (4), Tahiti (1), NE Australia (1), New Zealand (2). |
|
BURS/CONOPHORALIA |
1 (1) |
1 (3) |
1 (37) |
|
|
|
BURS/SCLEROPERALIA |
1 (9) |
1 (20) |
1 (47) |
|
|
|
|
2 (12) |
2 (26) |
2 (112) |
|
|
|
8. MICROGNATHOZOA |
MICROGNATHOZOA |
1 (1) |
1 (1) |
1 (2) |
|
|
9. SYNDERMATA |
PARAROTATORIA |
- (1) |
- (2) |
- (7) |
Seisonida occur Mediterranean region, including the Adriatic Sea, and the European part of the Atlantic, Sea of Okhotsk off the Sakhalin Island, California Antarctic Ocean and Kenya. |
|
MONOGONONTA |
27 (30) |
66 (115) |
569 (2,022) |
|
|
|
BDELLOIDEA |
3 (5) |
8 (20) |
38 (438) |
|
|
|
ARCHIACANTHOCEPHALA |
3 (4) |
4 (18) |
19 (187) |
|
|
|
PALAEACANTHOCEPHALA |
8 (17) |
13 (104) |
30 (884) |
|
|
|
EOACANTHOCEPHALA |
2 (4) |
8 (9) |
20 (255) |
|
|
|
POLYACANTHOCEPHALA |
4 (1) |
4 (1) |
2 (4) |
|
|
|
|
47 (62) |
103 (269) |
678 (3,797) |
|
|
|
10. ORTHONECTIDA |
ORTHONECTIDA |
- (2) |
- (5) |
- (26) |
Parasitics widely distributed W North America, Europe and Japan. |
|
11. DICYEMIDA/RHOMBOZOA |
DICYEMIDA |
- (3) |
- (9) |
- (147) |
Parasitics widely distributed worldwide. |
|
12. GASTROTRICHA |
MACRODASYIDA |
6 (12) |
9 (38) |
13 (393) |
|
|
CHAETONOTIDA |
4 (8) |
21 (40) |
76 (539) |
|
|
|
|
10 (20) |
30 (118) |
89 (932) |
|
|
|
13. PLATYHELMINTHES |
CATENULIDA |
3 (6) |
6 (12) |
42 (111) |
|
|
MACROSTOMORPHA |
3 (4) |
5 (24) |
17 (291) |
|
|
|
AMPLIMATRICATA |
20 (43) |
33 (188) |
61 (1,098) |
|
|
|
GNOSONESIMIDA |
- (1) |
- (1) |
- (6) |
|
|
|
RHABDOCOELA |
16 (38) |
41 (364) |
89 (1,838) |
|
|
|
PROSERIATA |
5 (11) |
18 (96) |
25 (498) |
|
|
|
ADIAPHANIDA |
12 (24) |
41 (209) |
211 (1,993) |
|
|
|
BOTHRIOPLANIDA |
1 (1) |
1 (1) |
1 (2) |
|
|
|
NEODERMATA |
157 (256) |
727 (2,657) |
1,675 (16,744) |
|
|
|
|
217 (383) |
873 (3,551) |
2,122 (22,214) |
|
|
|
14. ENTOPROCTA |
SOLITARIA |
1 (1) |
1 (6) |
7 (152) |
|
|
COLONIALES |
3 (4) |
5 (12) |
10 (52) |
|
|
|
|
4 (5) |
6 (18) |
17 (204) |
|
|
|
15. CYCLIOPHORA |
SYMBIIDAE |
- (1) |
- (1) |
- (3) |
Parasitics known from Atlantic coast of North America and Europe. |
|
16. NEMERTEA |
ARHYNCHOCOELA |
- (1) |
- (1) |
- (1) |
Known only from New Zealand. |
|
PALAEONEMERTEA |
2 (6) |
2 (11) |
2 (121) |
|
|
|
PILIDIOPHORA |
2 (7) |
5 (116) |
7 (534) |
|
|
|
HOPLONEMERTEA |
8 (35) |
12 (191) |
30 (771) |
|
|
|
|
12 (49) |
19 (319) |
39 (1,427) |
|
|
|
17. MOLLUSCA |
GASTROPODA |
265 (568) |
932 (7,848) |
2,737 (84,015) |
|
|
BIVALVIA |
80 (124) |
305 (1,668) |
629 (10,858) |
|
|
|
MONOPLACOPHORA |
- (4) |
- (7) |
- (31) |
|
|
|
CEPHALOPODA |
37 (54) |
79 (226) |
94 (987) |
|
|
|
SCAPHOPODA |
6 (17) |
19 (52) |
42 (617) |
|
|
|
APLACOPHORA |
6 (28) |
12 (108) |
22 (474) |
|
|
|
POLYPLACOPHORA |
7 (21) |
11 (142) |
37 (1,134) |
|
|
|
|
401 (816) |
1,358 (10,051) |
3,561 (98,119) |
|
|
|
18. ANNELIDA |
UNPLACED |
3 (11) |
10 (30) |
51 (275) |
|
|
PALEOANNELIDA |
2 (2) |
5 (6) |
18 (138) |
|
|
|
CHAETOPTERIDAE |
1 (1) |
4 (5) |
8 (84) |
|
|
|
AMPHINOMIDA |
2 (2) |
11 (28) |
20 (223) |
|
|
|
SIPUNCULA |
6 (6) |
12 (33) |
39 (153) |
|
|
|
EUNICIDA |
6 (9) |
44 (119) |
203 (1,515) |
|
|
|
PHYLLODOCIDA |
22 (26) |
170 (590) |
552 (5,076) |
|
|
|
PROTODRILIFORMIA |
4 (5) |
6 (15) |
9 (107) |
|
|
|
ORBINIIDA |
2 (4) |
10 (52) |
36 (345) |
|
|
|
CIRRATULIFORMIA |
7 (8) |
33 (134) |
74 (1,171) |
|
|
|
SIBOGLINIDAE |
1 (1) |
3 (34) |
5 (214) |
|
|
|
SABELLIDA |
3 (4) |
47 (144) |
88 (1,408) |
|
|
|
SABELARIIDA |
1 (1) |
6 (14) |
19 (156) |
|
|
|
SPIONIDA |
6 (8) |
27 (61) |
114 (813) |
|
|
|
CAPITELLIDA / ECHUIRA |
4 (7) |
38 (134) |
81 (711) |
|
|
|
SCALIBREGMATIDAE / TRAVISIDAE |
2 (2) |
5 (18) |
17 (193) |
|
|
|
OPHELIIDAE |
1 (1) |
7 (10) |
32 (180) |
|
|
|
ARENICOLIDA |
1 (1) |
2 (6) |
4 (25) |
|
|
|
TEREBELLIFORMEA |
3 (3) |
28 (76) |
80 (845) |
|
|
|
AEOLOSOMATIDA / HRABEIELLIDA |
1 (2) |
1 (4) |
11 (49) |
|
|
|
CLITELLATA |
20 (59) |
111 (952) |
416 (9,370) |
|
|
|
|
98 (163) |
580 (2,465) |
1,877 (23,051) |
|
|
|
19. BRACHIOPODA |
CRANIIFORMEA |
1 (1) |
1 (3) |
1 (11) |
|
|
LIGULIFORMEA |
2 (2) |
3 (6) |
3 (25) |
|
|
|
RHYNCHONELLIFORMEA |
5 (28) |
5 (112) |
7 (373) |
|
|
|
|
8 (31) |
9 (121) |
11 (409) |
|
|
|
20. BRYOZOA |
GYMNOLAEMATA |
91 (201) |
205 (859) |
460 (6,198) |
|
|
STENOLAEMATA |
10 (27) |
15 (130) |
34 (729) |
|
|
|
PHYLACTOLAEMATA |
4 (8) |
4 (18) |
26 (110) |
|
|
|
|
105 (236) |
224 (1,007) |
520 (7,037) |
|
|
|
21. PHORONIDA |
PHORONIS / PHORONOPSIS |
1 (1) |
1 (2) |
5 (15) |
|
|
22. LORICIFERA |
LORIFICERA |
1 (2) |
1 (11) |
1 (47) |
|
|
23. KINORHYNCHA |
CYCLORHAGIDA |
2 (5) |
2 (17) |
4 (242) |
|
|
ALLOMALORHAGIDA |
3 (5) |
4 (16) |
4 (122) |
|
|
|
|
5 (10) |
6 (33) |
8 (364) |
|
|
|
24. PRIAPULIDA |
MEIOPRIAPULIDAE |
- (1) |
- (1) |
- (1) |
Collected in Bengal Bay, Fiji and South Korea. |
|
TUBILUCHIDAE |
- (1) |
- (1) |
- (10) |
Widespread in Bermuda, Florida to Curazao, Canary Islands, Sweden, continental Spain, Italy, White Sea and Barents Sea, Red Sea, Japan, Philippines, NE Australia, Vanuatu and Hawaii. |
|
|
CHAETOSPHANIDAE |
- (1) |
- (1) |
- (2) |
W Mediterranean and Andaman Sea. |
|
|
MACROPRIAPUULIDA |
1 (2) |
1 (4) |
1 (10) |
|
|
|
|
1 (5) |
1 (7) |
1 (23) |
|
|
|
25. NEMATOMORPHA |
NECTONEMATOIDA |
1 (1) |
1 (1) |
1 (5) |
|
|
GORDIOIDA |
2 (2) |
6 (20) |
22 (466) |
|
|
|
|
3 (3) |
7 (21) |
22 (471) |
|
|
|
26. NEMATODA |
ENOPLEA |
32 (92) |
82 (794) |
179 (7,706) |
|
|
CHROMADOREA |
92 (242) |
489 (2,486) |
1,385 (15,921) |
|
|
|
|
124 (334) |
571 (3,280) |
1,564 (23,627) |
|
|
|
27. TARDIGRADA |
MESOTARDIGRADA |
- (1) |
- (1) |
- (1) |
Known only from S Japan, a nomina dubium. |
|
EUTARDIGRADA |
7 (17) |
20 (82) |
43 (921) |
|
|
|
HETEROTARDIGRADA |
12 (15) |
29 (79) |
55 (568) |
|
|
|
|
19 (33) |
49 (162) |
98 (1,490) |
|
|
|
28. ONYCHOPHORA |
PERIPATIDAE |
1 (1) |
4 (13) |
22 (82) |
|
|
PERIPATOPSIDAE |
- (1) |
- (41) |
- (138) |
A family highly centered in eastern Australia, but also displaying notable diversity in South Africa and neighboring Lesotho, with residual diversity in central Chile, New Zealand, New Guinea, and nearby islands. |
|
|
|
1 (2) |
4 (54) |
22 (220) |
|
|
|
29. ARTHROPODA |
PYCNOGONIDA |
11 (11) |
19 (108) |
74 (1,476) |
|
|
ARACHNIDA |
404 (859) |
2,178 (13,536) |
8,394 (110,209) |
|
|
|
CHILOPODA |
10 (17) |
34 (445) |
148 (3,425) |
|
|
|
PAUROPODA |
4 (12) |
11 (56) |
63 (1021) |
|
|
|
SYMPHYLA |
2 (2) |
4 (14) |
12 (243) |
|
|
|
DIPLOPODA |
25 (178) |
171 (2,413) |
584 (14,026) |
|
|
|
OLIGOSTRACA |
25 (51) |
190 (796) |
453 (5,823) |
|
|
|
THECOSTRACA |
17 (60) |
47 (331) |
73 (2,214) |
|
|
|
MALACOSTRACA |
290 (719) |
917 (7,367) |
2,240 (46,560) |
|
|
|
CEPHALOCARIDA |
1 (1) |
2 (5) |
2 (12) |
|
|
|
BRANCHIOPODA |
19 (34) |
74 (172) |
189 (1,847) |
|
|
|
COPEPODA |
78 (253) |
290 (2,479) |
867 (15,665) |
|
|
|
REMIPEDIA |
- (8) |
- (12) |
- (28) |
||
|
COLLEMBOLA |
21 (33) |
131 (776) |
618 (9,092) |
|
|
|
DIPLURA |
4 (10) |
15 (143) |
31 (866) |
|
|
|
PROTURA |
2 (7) |
13 (77) |
27 (821) |
|
|
|
INSECTA |
703 (1,445) |
15,939 (114,915) |
91,241 (1,077,159) |
|
|
|
|
1,616 (3,700) |
20,035 (143,645) |
105,016 (1,290,487) |
|
|
|
30. HEMICHORDATA |
ENTEROPNEUSTA |
2 (4) |
5 (24) |
10 (117) |
|
|
PTEROBRANCHIA |
1 (3) |
1 (3) |
1 (32) |
|
|
|
|
3 (7) |
6 (27) |
11 (149) |
|
|
|
31. ECHINODERMATA |
CRINOIDEA |
8 (54) |
16 (212) |
21 (788) |
|
|
HOLOTHURIOIDEA |
16 (35) |
42 (283) |
72 (1,840) |
|
|
|
ECHINOIDEA |
23 (65) |
42 (294) |
55 (1,032) |
|
|
|
OPHIUROIDEA |
25 (39) |
61 (291) |
138 (2,202) |
|
|
|
ASTEROIDEA |
25 (46) |
49 (392) |
70 (2,032) |
|
|
|
|
97 (239) |
210 (1,472) |
356 (7,894) |
|
|
|
32. CEPHALOCHORDATA |
BRACHIOSTOMIDAE |
1 (1) |
2 (3) |
3 (31) |
Epigonichthys cultellus Peters, 1877, occur in coast of Thailand, south Japan, Hawaii and W Pacific Ocean, mainly around Australia. |
|
33. TUNICATA |
ASCIDIACEA |
20 (34) |
61 (221) |
180 (3,006) |
|
|
APPENDICULARIA |
3 (5) |
10 (21) |
44 (70) |
|
|
|
|
23 (39) |
71 (241) |
224 (3,076) |
|
|
|
34. CRANIATA |
MYXINI |
1 (1) |
3 (6) |
5 (91) |
|
|
PETROMYZONTI |
- (3) |
- (10) |
- (48) |
|
|
|
ELASMOBRANCHII |
37 (65) |
83 (212) |
181 (1,256) |
|
|
|
HOLOCEPHALI |
3 (3) |
4 (6) |
4 (60) |
|
|
|
CLADISTII |
- (1) |
- (2) |
- (14) |
|
|
|
ACTINOPTERI |
222 (538) |
1,352 (5,094) |
5,030 (36,042) |
|
|
|
COELACANTHII |
- (1) |
- (1) |
- (2) |
|
|
|
DIPNEUSTII |
1 (3) |
1 (3) |
1 (6) |
|
|
|
AMPHIBIA |
27 (79) |
121 (597) |
1,253 (9,007) |
|
|
|
RHYNCHOCEPHALIA |
- (1) |
- (1) |
- (1) |
A single reptile restricted of small islands aroung North Island in New Zealand. |
|
|
SQUAMATA |
25 (70) |
165 (1,179) |
778 (12,324) |
|
|
|
TESTUDINES |
8 (14) |
19 (96) |
36 (376) |
|
|
|
CROCODILIA |
1 (3) |
3 (9) |
7 (28) |
|
|
|
AVES |
81 (254) |
650 (2,302) |
1,712 (11,887) |
|
|
|
MAMMALIA |
49 (153) |
248 (1,360) |
778 (6,871) |
|
|
|
|
413 (1,112) |
2,558 (10,638) |
9,594 (76,536) |
|
|
|
TOTAL |
3,499 (8,139) |
27,509 (182,551) |
127,776 (1,596,517) |
|
|
This table will always be modified and updated when more accurate and viable data becomes available - and unfortunately many more recent works, which could distort the stability of the data, have been omitted. It should be noted that the numbers used in this blog and, therefore, in the table, are based on checklists, some of them old, manual counts subject to errors and estimates that are not so precise. Thus, the numbers posted do not include many new species, but it is, within the scope of this research, the most detailed numbers that could be obtained.
6 CANONIC LINEAGES UNKNOWN IN BRAZIL
The 22 canonic lineages not recorded in Brazil: Polyplacotomia [1], Polypodiozoa [2], Xenoturbellida [3], Nemertodermatida [4], Filospermoidea [5], Seisonida [6], Orthonectida [7], Dicyemida [8], Gnosonesimida [9], Arhynchocoela [10], Cycliophora [11], Monoplacophora [12], Meiopriapulus [13], Tubiluchus [14], Maccabeus [15], Mesotardigrada [16], Peripatopsidae [17], Remipedia [18], Petromyzonti [19], Cladistii [20], Coelacanthi [21] and Rhynchocephalia [22].
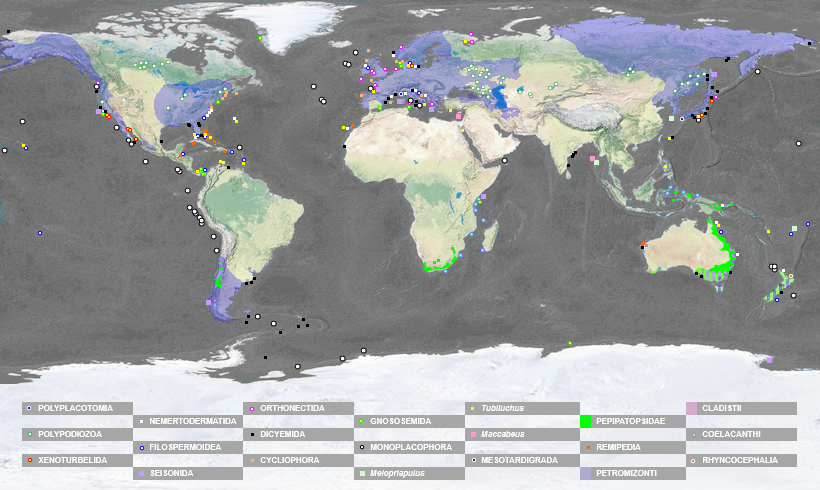
HIGH ACCURATE MAP OF THE DISTRIBUTION OF 21 0FF 22 CANONIC METAZOA LINEAGES NOT RECORDED IN BRAZIL (EXCEPT ARHYNCHOCOELA, IN APRIL 17, 2025):
Polyplacotomia (CB),
Polypodiozoa (JAI),
Xenoturbellida (NAT | BMC),
Nemertodermatida (BJZ),
Gnosonesimida (GBIF | MS | Worms/G. borealis | VLAAMS),
Meiopriapulus (ZA),
Tubiluchus (ZA),
Maccabeus (ZA),
Seisonida (JMBAUK | ZS),
Cycliophora (ME),
Monoplacophora (ZSM | ZOO | RUT | MR | VEL | JMS | GBIF),
Dicyemida (Zoo | SP | FP | JP),
Orthonectida (Wiki | IB),
Peripatopsidae (GBIF),
Mesotardigrada (ZS),
Remipedia (RD),
Petromyzonti (FAO | RFBF | NCBI),
Cladistii (IEF),
Coelacanthi (NAT) and
Rhynchocephalia (WIKI). In some regions with a high density of records, the map may show slight deviations to enhance the visual representation of the taxon's occurrence: Mediterranean Sea, northern Europe near Denmark, Sweden and Norway, and California.
1. CTENOPHORA ‣ (35:55/)202 spp. worldwide (Catalogue of Life/Ctenophora) in two groups: Tentaculata (34:53/158) and Nuda (1:2/44, in Beroidae). (9:11/)14 spp. occur in Brazilian coasts (CTFB/Ctenophora) across five orders representing both orders. Oliveira et al. (Zootaxa, 2016) lists 20 ctenophores in waters of South America in 11 families. Only the orders Ganeshida, Cambojiida, and Cryptolobiferida — restricted to SE Asia — are not recorded from from the continent. Mexico hosts (13:17/)30 spp. (2 in the Gulf of Mexico, 7 in the Mexican Caribbean Sea, 25 in the Gulf of California, 11 in the E Tropical Pacific, and only 1 are known in the NE Pacific — Puente-Tapia et al., RSMS, 2021).
The families Thalassocalycidae (sole representative of the order Thalassocalycida in South America) and Mertensiidae are recorded in Chilean and Argentine waters but are not recorded from from Brazil.
Several observations of large, globular Ctenophora with long filamentous tentacles and lacking oral lobes from 10,040 m in the Kermadec Trench (SW Pacific Ocean) are the deepest worldwide for this phyllum (Jamieson, AJ et al., Marine Biology, 2023).
Some checklist worldwide includes Cnidaria and Ctenophora from Malta (155 Cnidaria, 5 Ctenophora, Mifsud, Bulletin of the National Museum of Natural History, 2025) and Cnidaria and Ctenophora from Turkyie (195 in Cnidaria, 7 in Ctenophora, Çinar, ME et al, Turkish Journal of Zoology, 2014).
▉ LAST UPDATE OF CTENOPHORA IN JANUARY/FEBRUARY 2026
2. PORIFERA ‣ (173:889/)10,206 spp. worldwide in four classes (Catalogue of Life), (91:229/)662 in Brazil, in all classes. Catalogue of Brazilian Porifera (Museu Nacional, Guilherme Muricy et al., 2011) recognizes 53 spp. of freshwater Porifera in Brazil, some endemic.
For Brazilian deep-sea sponges, see Hajdu & Lopes (110 spp., Checklist of Brazilian Deep-sea Sponges, 2007), where Thenea fenestrata Sollas, 1886 is the deepest Porifera from Brazil (3138m), along Alagoas state.
All data presented below follow Catalogue of Life/Porifera at the global level and CTFB/Porifera for Brazil.
Some checklist worldwide includes Porifera from Caribbean coast of Colombia (81 spp., Velderrama & Zea, Revista de la Academia Colombiana de Ciencias Exactas, 2013) and Porifera from Greece (65:111/215 spp., Voultsiadou, E et al., Biodiversity Data Journal, 2016).
DEMOSPONGIAE
(119:626/)8,398 spp. worldwide, unique lineage also in freshwater, (70:187/)539 in Brazil. Mexico includes 517 spp. of Demospongiae (Carballo et al., Revista Mexicana de Biodiversidad, 2014).
All (6:45/)219 spp. (or 268 in Rasbold et al., Front. Ecol. Evol., 2023) freshwater sponges belongs Spongillida clade of Demospongiae (Balian, 2008). All six freshwater families are fully freshwater. In the Neotropics, the most rich region of this sponges (followed by the Palearctic-59 and Afrotropics-49), freshwater sponges are (3:23/)65 spp. (Balian, 2008), or 77 (Rasbold et al., 2023), in Potamolepidae (Africa, South America, New Caledonia, Fiji, 3/11 in this region | Copeland et al., Zootaxa, 2015), Spongillidae (14/35 in this region) and Metaniidae (5/17 in this region).
The three freshwater sponge families unknowns in South America are Lubomirskiidae (10, endemic to Lake Baikal, S Russia), Metschnikowiidae (1, Caspian Lake) and Malawispongiidae (6, Tanganyika and Malawi Lakes in Africa, Ohrid in North Macedonia and E Albania, Kinneret Lake in Israel and Syria, and Poso Lake in C Sulawesi).
CALCAREA
(26:96/)922 spp. worldwide. Brazil has (10:23/)84 spp.
HEXACTINELLIDA
(26:155/)750 spp. worldwide. Brazil has (9:15/)22 spp. The deepest record for the class Hexactinellida belongs at least two different morphotypes observed at 7180m water depth in the Java Trench on Indian Ocean (Marchiò et al, Marine Biology, 2025).
HOLOSCLEROMORPHA
(2:12/)136 spp. worldwide. Brazil includes (4/)17 spp., in both families of this class, Plakinidae (1/3) and Oscarellidae (3/14).
▉ LAST UPDATE OF PORIFERA IN JANUARY/FEBRUARY 2026
3. PLACOZOA ‣ simple, morphologically indistinguishable marine animals. The most recent phylogenetic analysis of Placozoa, by Tessler et al. (Frontiers in Ecology and Evolution, 2022), recognizes two groups: the class Polyplacotomia (containing a single species) and Uniplacotomia (comprising 22 species in 7 genera across 4 families, one of which — along with 4 genera — remains unnamed).
Of the 23 species identified, only four have been formally described (SEE): Trichoplax adhaerens Schulze, 1883 (amoeboid, widely distributed in coastal areas, and the only species recorded in Brazil, Morandino et al., Zoologischer Anzeiger, 2006), Hoilungia hongkongensis Eitel, Schierwater & Wörheide, 2018 (amoeboid, known from a mangrove area in Hong Kong, in Eitel et al., Plos Biology, 2018), Cladtertia collaboinventa Tessler et al., 2022 (ameboid, known from aquarium environments), and Polyplacotoma mediterranea Osigus et al., 2019 (a ramified form, known only from Alassio, Italy, by Osigus & Schierwater, Current Biolgy, 2019). For a global map of Trichoplax collection sites, see Eitel et al. (Plos One, 2013, 2013).
▉ LAST UPDATE OF PLACOZOA IN JANUARY/FEBRUARY 2026
4. CNIDARIA ‣ (487:2,214/)16,788 spp. distributed across eight subgroups (Catalogue of Life, sectioned data), (173:416/)915 spp. recorded in Brazil, numbers based on a compilation of the data below analyzed by class. For the global status of medusae, see Jankowski (Hydrobiologia, 2001). In Pacific Mexico, 423 spp. are recorded across Cubozoa, Scyphozoa, and Hydrozoa, and 35 spp. of Scyphozoa occur in all Mexican coasts (Gasca & Loman-Ramos, Revista Mexicana de Biodiversidad, volume 85, 2014 | Estrada-González, MC et al., Journal of Natural History, 2023).

PHYLOGENY OF CNIDARIA EXCEPT MYXOZOA AND POLYPODIOZOA
We recognize (4:7/)42 spp. of freshwater non parasitic Cnidaria, all in Hydrozoa (Balian, 2008 | Deserti et al., Revista de Biologia Tropical, 2023, Polypodiozoa and Myxozoa are mainly freshwater parasitics), in Hydra (simple, solitary polyps lacking medusae, Hydridae), Cordylophora (colonial hydroids, Cordylophoridae), Pachycordyle (colonial hydroids, Cordylophoridae), Craspedacusta (medusae, Olindiidae), Limnocnida (medusae, Olindiidae), Calpasoma (small polyp, Olindiidae), and Velkovrhia (Velkovrhia enigmatica Matjašić & Sket, 1971, endemic to the Dinaric karst region of the Balkan Peninsula, where it has been reported from five caves: three in Slovenia, one in Croatia, and one in Bosnia, as noted by Magmajster, Natura Sloveniae, 2003), Bougainvilliidae).
There is some controversy regarding the bizarre polyps of Craspedacusta sowerbyi, with some authors even suggesting that a distinct species may exist — Calpasoma dactylopterum Fuhrmann, 1939, from the same family. The latter would only occur in the form of solitary polyps with tentacles (whereas C. sowerbyi has branched, tentacle-less polyps). It has been recorded in Brazil (São Paulo), Argentina, and Uruguay (Planeta Invertebrados).
In the Neotropical region, (4/)14 freshwater species of Hydrozoa are known (Deserti et al., Revista de Biologia Tropical, 2023 | Deserti et al., Zoologischer Anzeiger, 2015): Calpasoma dactylopterum Fuhrmann, 1939 (Olindiidae, SE Brazil, NE Argentina, and Uruguay), Craspedacusta sowerbii Lankester, 1880 (Olindiidae, Argentina, Uruguay, Chile, Brazil, Venezuela, Mexico, Panama, Belize, and Costa Rica), Cordylophora caspia Pallas, 1771 (Cordylophoridae, Argentina, Uruguay, Chile, SE Brazil, N Colombia, and E Mexico), and Hydra (11 in region, mainly found in Argentina-4, Brazil-4/3 endemic, Mexico-4, Chile-4, and Paraguay-4).
Checklists worldwide of Cnidaria includes Medusozoa from Portugal (272 spp., being 254 hydrozoans, 15 scyphozoans, 2 staurozoans, and 1 cubozoan, Rodruiguez, T et al., Regional Studies in Marine Science, 2020), Medusozoa from Mexico (169 spp., Segura-Puertas & Suárez-Morales, Zootaxa, 2011), Octocorallia from Azores (101 spp., Íris, S et al., Zootaxa, 2019), Cnidaria from Mediterranean Morocco (104 spp., Mghili, B et al., Regional Studies in Marine Science, 2024), Cnidaria and Ctenophora from Malta (155 Cnidaria, 5 Ctenophora, Mifsud, Bulletin of the National Museum of Natural History, 2025), and Cnidaria and Ctenophora from Turkyie (195 in Cnidaria, 7 in Ctenophora, Çinar, ME et al, Turkish Journal of Zoology, 2014).
TAXONOMY
HEXACORALLIA
Assignated as Anthozoa in Catalogue of Life. (155:856/)4,720 living spp. worldwide (Catalogue of Life), (45:145/)227 in Brazil (CTFB/Cnidaria). For details of Isaurus tuberculatus Gray, 1828 in Brazil, see Lima et al. (CheckList, 2022). For some notes for Actiniaria in Brazil, see Targino, A.K. et al. (Zootaxa, 2025).
OCTOCORALLIA
(97:480/)4,103 spp. worldwide (Catalogue of Life), (21:48/)76 in Brazil (CTFB/Cnidaria). The traditionally recognized orders of Octocorallia included Alcyonacea, Pennatulacea, and Helioporacea (unknown in Brazil); however, they are currently rearranged into only two clades, Scleralcyonacea and Malacalcyonacea.
MYXOZOA
(31:75/)3,131 spp. worldwide (Catalogue of Life), (11:19/)221 in Brazil (CTFB/Cnidaria). Vidal (Thesis, 2017) reports (15:36/)495 spp. in the New World. According to this source, Brazil lacks the genera Palliatus, Myxoproteus, Myxodavisia, Bipteria, Parvicapsula, Myxobiliatus, Zschokkella, Auerbachia, Renispora, Pseudolantospora, and Alatospora, as well as the South American family Alatosporidae. Myxozoa appears no has described species in Ecuador, Colombia, and Venezuela, and is represented by 37 spp. in Mexico (Alama-Bermejo, Scientific Reports, 2023).
Malacosporea, with a single family, Saccosporidae (2/3 spp., from North America, Europe and Borneo, SEE), is sometimes treated as a distinct class of Myxozoa (Wikipedia). Here, we address it under the latter name, but there is a possibility of considering it valid in the future.
POLYPODIOZOA
The position of Polypodiozoa within Cnidaria is unstable, due to imprecision in molecular data. Here, as in many references, it is accepted as its own monotypic class, most often recovered as the sister group to Myxozoa; in other sources, it is placed within the core of Hydrozoa; and in some studies, it is even speculated not to belong to Cnidaria at all (Novosolov et al, Genome Biology and Evolution, 2022).
Only one egg fish-parasitic species (unique intracelular parasitic in Metazoa), Polypodium hydriforme Ussov, 1885, from parts of Russia (Volga, Kama, Don, north Dvina, Sulak and Kuban in E Europe, Selenga in Siberia, and Amur and Sakhalin island in NE Asia), Romania (Danube), Kazakhstan (Ural and Syr Darya), Moldavia (Dnestr), Ukraine (Don), China (only Lake Khanka), Iran (Sefid Rud), Canada (Nelson, Saskatchewan and St. John rivers), and E & W USA (Raikova, Journal of Applied Ichthyology, 2002).
STAUROZOA
(8:16/)55 spp. worldwide (Catalogue of Life), two spp. in Brazil (CTFB/Cnidaria), Calvadosia capensis (Carlgren, 1938) and C. corbini (Larson, 1980), in Kishinouyeidae. For additional information, including checklists and a global distribution map of these species, see Miranda et al. (Marine Biodiversity, 2017). In the Neotropical region, this class is known only from Mexico (3, SEE), Puerto Rico, Brazil, Argentina, and Chile. The third South American species, Haliclystus antarcticus Pfeffer, 1889 (Haliclystidae), is unknown from Brazil.
CUBOZOA
(8:21/)54 spp. worldwide (Catalogue of Life), (4:4/)4 in Brazil in Brazil (CTFB/Cnidaria).
HYDROZOA
Hydroids, hydra-like animals. (157:683/)4,404 spp. worldwide (Catalogue of Life), (79:190/)365 in Brazil (CTFB/Cnidaria). This class includes Dendrogramma (SEE), an enigmatic animal from coasts of southern Australia that has even been speculated to belong to his own phylum (O´Hara et al., Current Biology Magazine, 2016).
Observations of Pectis cf. profundicola (Rhopalonematidae) from both lander and submersible dives at 10,063 and 10,040m in the Philippine Trench (NW Pacific Ocean) is the first record of Hydrozoa below 10,000 m and the deepest worldwide (Jamieson, AJ et al., Marine Biology, 2023).
Bougainvillidae has (6/)12 spp. in Brazil, all marine.
■ endemic families in New World: Tottonophyidae (1/1, Siphonophora, U.S.A).
SCYPHOZOA
(30:82/)320 spp. worldwide (Catalogue of Life), (12:14/)20 in Brazil (CTFB/Cnidaria). Only one family in South America does not occurs in Brazil: Phacellophoridae, with one species from Pacific and Argentinian coasts.
▉ LAST UPDATE OF CNIDARIA IN JANUARY/FEBRUARY 2026
5. XENACOELOMORPHA ‣ small, flat and worm-like in marine and sometimes brackish water environments, on the sediments. Three clades with (19:113/)454 spp. worldwide, (9:24/)33 in Brazil, all only in Acoela. All data presented below follow Catalogue of Life/Xenacoelomorpha at the global level and CTFB (CTFB/Acoelomorpha) for Brazil, with additions of some species, genera and families at Brazilian count.
XENOTURBELLIDA
Six species in Xenoturbella (Wikipedia): one collected off the coast of SW Sweden, which is the type species; one species found at two sites along the coast of Japan (Nakano et al., BMC Evol Biol., 2017); and four species collected along the Pacific coast of North America, ranging from California (2) to the Gulf of California in Mexico (3), with one species common to both areas (Rouse et al., Nature, 2016). It has never been recorded in Brazil.
NEMERTODERMATIDA
(2:6/)18 spp. worldwide, known only from a few distinct sampling spots: Sweden, Norway, Canary Islands, Belgium, E coast of North America, Bermudas, Adriatic and Mediterranean seas, New Guinea, Australia (Queesland) and New Zealand (Sterrer, Belgian Jounal of Zoology, 1998).
ACOELA
(16:106/)430 spp. worldwide, and (9:24/)33 spp. in Brazil. Only two species worldwide are freshwaters: Limonoposthia polonica Kolasa et Faubel, 1974 from lakes from Polonia, and Oligochoerus limnophilus Ax & Dörjes, 1966 from southern UK, southern France, N & C Germany, Netherlands and W Romania (Vila–Farré et al., Arxius de Miscellània Zoològica, 2013).
▉ LAST UPDATE OF XENACOELOMORPHA IN JANUARY/FEBRUARY 2026
GNATHIFERA
6. CHAETOGNATHA ‣ (11:35/)134 spp. worldwide (Catalogue of Life/Chaetognatha) in two orders: Aphragmophora (6:23/66) and Phragmophora (5:12/68), and (5:14/)25 in Brazil (CTFB/Chaetognatha) in Aphragmophora (3:12/22) and Phragmophora (2:2/3). A checklist of the world Sagittidae is provided by A.P. Kassatkina (Zoosystematica Rossica, 2007).
▉ endemic families in New World: Bathybelidae (1/1, U.S.A).
▉ LAST UPDATE OF CHAETOGNATHA IN JANUARY/FEBRUARY 2026
7. GNATHOSTOMULIDA ‣ (12:26/)112 spp. within two orders. Despite the possibility that many Gnathostomulida species are cosmopolitan — largely due to the wide distribution of several of them — only a few locations worldwide have been investigated in detail. For the data below, we refer to the combined works of Gnathostomulda worldwide (Sørensen & Sterrer, BOOK, 2022), in Caribbean (Sterrer, Book, 1997), Gulf of Mexico (Sørensen and Sterrer, Chapter 26, 2005), Sweden, North Ireland and Croatia (Sterrer, BOOK, 1969), Australia and Papua New Guinea (Sterrer, free papper, 2001), New Zealand, Tonga and Fiji (8/18, Sterrer, Zoologica Scripta, 1991), New Zealand alone (4/10, Sterrer, Zootaxa, 2006), Hawaii (3/8, Sterrer, Zoologica Scripta, 1991) and Tahiti (4/9, Sterrer, Zoologica Scripta, 1991).
Apart from the references above, here we include two records of Gnathostomulida in Brazil: Gnathostomula sp. and Austrognathia sp., both cited for Araçá Bay, São Paulo (Amaral et al., Biota Fapesp-Araçá, 2018), both undescribed. Sterrer (Book, 1997) cites records of Gnathostomula axi Kirsteuer, 1956, off coast of Venezuela in South America, and this phyllum remains unknown in Mexico.
World diversity: NE Pacific (1:2/2), Galapagos (4:4/4), NW Atlantic (1:1/1), Bermuda (4:5/10, endemic family Problongnathiidae), Bahamas (4:4/4), SE USA (9:13/20), Caribbean (4:4/4), Puerto Rico (1:1/2), Barbados (1:1/1), Belize (5:6/14, endemic family Paucidentulidae), Panama (4:5/6), Brazil (2:2/2), Canary Islands (4:4/5), W Europe (1:1/1), Ireland (3:3/7), Mediterranean Region (1:1/1), Denmark (2:2/2, endemic family Rastrognathiidae), Sweden (3:3/12), Croatia (4:4/4), Barents (1:1/1), South Africa (1:1/1), Madagascar (1:1/1), Reunion (1:1/1), Red Sea (1:1/1), Maldives (1:1/1), Hong Kong (2:2/2), Thailand (1:1/1), Papua New Guinea (1:1/1), Australia (6:7/10), New Zealand (5:5/9), New Caledonia (1:1/1), Fiji (7:8/13), Tahiti (5:5/5), Hawaii (3:4/8).
FILOSPERMOIDA
(2:3/)28 spp. in Bermuda (2:3/8), Bahamas (1:1/1), SE USA (2:3/10), Puerto Rico (1:1/2), Belize (2:3/11), Panama (2:3/4), Canary Islands (1:1/2), Ireland (2:2/6), Sweden (2:2/11), Croatia (1:1/2), Australia (2:2/5), New Zealand (2:2/6), Fiji (2:3/6), Tahiti (2:2/2), Hawaii (2:3/7).
HAPLOGNATHIIDAE
A single genus, Haplognathia (10) — Bermuda (1:1/3), SE USA (1:1/5), Belize (1:1/4), Panama (1:1/1), Canary Islands (1:1/2), Ireland (1:1/4), Sweden (1:1/7), Croatia (1:1/2), Australia (1:1/4), New Zealand (1:1/4), Fiji (1:1/1), Tahiti (1:1/1), Hawaii (1:1/3).
Haplognathia (10): H. asymmetrica (Bermuda, Belize, Hawaii, North Carolina, New Zealand, NE Australia), H. belizensis (Belize), H. filum (Kristineberg in Sweden), H. gubbarnorum (North Carolina, Kristineberg in Sweden, Portaferry in N Ireland, Istria in Croatia, New Zealand, NE Australia), H. lunulifera (Belize, North Carolina, Kristineberg in Sweden, Portaferry in N Ireland), H. rosea (Bermuda, Florida, Belize, Panama, North Carolina, Canary Islands, Kristineberg in Sweden, Portaferry in N Ireland, Fiji, Tahiti, New Zealand, NE Australia), H. ruberrima (Bermuda, North Carolina, Kristineberg in Sweden, Canary Islands, Istria in Croatia, Hawaii, New Zealand, NE Australia), H. rubromaculata (Kristineberg in Sweden), H. rufa (Hawaii) and H. simplex (Kristineberg in Sweden, Portaferry in N Ireland).
PTEROGNATHIIDAE
(2/)18 spp. in Cosmognathia (4) and Pterognathia (14) — Bermuda (1:2/5), Bahamas (1:1/1), SE USA (1:2/5), Puerto Rico (1:1/2), Belize (1:2/7), Panama (1:2/3), Ireland (1:1/2), Sweden (1:1/4), Australia (1:1/1), New Zealand (1:1/2), Fiji (1:2/5), Tahiti (1:1/1), Hawaii (1:2/4)..
Cosmognathia (4): C. aquila (Bermuda, Belize, Panama), C. arcus (Bermuda, Florida, Belize, Puerto Rico, Fiji, Hawaii), C. bastillae (Fiji) and C. manubrium (Bermuda, Belize, Puerto Rico, Panama, Hawaii, Tahiti).
Pterognathia (14): P. alcicornis (Belize), P. atrox (Kristineberg in Sweden, Portaferry in N Ireland), P. crocodilus (Bermuda, Florida, Belize, North Carolina, Fiji), P. ctenifera (Bermuda, Florida, Belize, Panama, North Carolina, Fiji, Hawaii), P. hawaiiensis (Hawaii), P. grandis (Bahamas), P. meixneri (Kristineberg in Sweden), P. portobello (New Zealand), P. pygmaea (North Carolina), P. sica (NE Australia), P. sorex (Kristineberg in Sweden, North Carolina), P. swedmarki (Belize, Kristineberg in Sweden, Portaferry in N Ireland), P. tuatara (New Zealand), P. ugera (Bermuda, Belize, Panama, Puerto Rico, Tahiti, New Zealand) and P. vilii (Fiji).
BURSOVAGINOIDA
(10:23/)84 spp. in two high groups in NE Pacific (1:2/2), Galapagos (4:4/4), NW Atlantic (1:1/1), Bermuda (2:2/2, endemic family Problongnathiidae), Bahamas (3:3/3), SE USA (7:10/10), Caribbean (4:4/4), Barbados (1:1/1), Belize (3:3/3, endemic family Paucidentulidae), Panama (2:2/2), Canary Islands (3:3/3), W Europe (1:1/1), Ireland (1:1/1), Mediterranean Region (1:1/1), Denmark (2:2/2, endemic family Rastrognathiidae), Sweden (1:1/1), Croatia (3:3/3), Barents (1:1/1), South Africa (1:1/1), Madagascar (1:1/1), Reunion (1:1/1), Red Sea (1:1/1), Maldives (1:1/1), Hong Kong (2:2/2), Thailand (1:1/1), Papua New Guinea (1:1/1), Australia (4:5/5), New Zealand (3:3/3), New Caledonia (1:1/1), Fiji (5:5/7), Tahiti (3:3/3), Hawaii (1:1/1).
CONOPHORALIA
A single family worldwide, in Galapagos (2:2/2), Bermuda (1:1/1), Bahamas (1:1/1), SE USA (2:2/2), Caribbean (2:2/2), Barbados (1:1/1), Canary Islands (1:1/1), Ireland (1:1/1), Croatia (2:2/2), South Africa (1:1/1), Madagascar (1:1/1), Reunion (1:1/1), Red Sea (2:2/4), Hong Kong (2:2/2), Thailand (1:1/1), Australia (1:1/1), New Zealand (2:2/2), Tahiti (2:2/2), Fiji (2:2/4), Hawaii (1:1/1).
AUSTROGNATHIIDAE
(3/)37 spp. in Austrognatharia (19, Red Sea and Fiji two each, Galapagos, Bermuda, Caribbean, Barbados, Canary Islands, Adriatic, South Africa, Madagascar, Mauritius, Reunion, Hong Kong, Thailand, NE Australia, New Zealand and Tahiti one each), Austrognathia (16, Red Sea, NW Atlantic and Fiji two each, Hawaii, Galapagos, Florida, Bahamas, Caribbean, N Ireland, Hong Kong, New Zealand and Taihiti one each) and Triplignathia (2, one in Croatia, another in North Carolina, USA). One undescribed species in Brazil, a undescribed species collected in Araça Bay, São Paulo state.
SCLEROPERALIA
Nine families and (20/)47 spp. worldwide, only one in Brazil, a undescribed species collected in Araça Bay, São Paulo state. World diversity: NE Pacific (1:2/2), Galapagos (2:2/2), NW Atlantic (1:1/1), Bermuda (1:1/1, endemic family Problongnathiidae), Bahamas (2:2/2), SE USA (5:8/8), Caribbean (2:2/2), Belize (3:3/3, endemic family Paucidentulidae), Panama (2:2/2), Canary Islands (2:2/2), W Europe (1:1/1), Mediterranean Region (1:1/1), Denmark (2:2/2, endemic family Rastrognathiidae), Sweden (1:1/1), Adriatic (1:1/1), Barents (1:1/1), Red Sea (1:1/1), Maldives (1:1/1), Papua New Guinea (1:1/1), Australia (3:4/4), New Zealand (1:1/1), New Caledonia (1:1/1), Fiji (3:3/3), Tahiti (1:1/1).
AGNATHIELIIDAE
(2/)3 spp. in Agnathiella (2, one in Florida Keys, one in Lizard Island in Australia, Fiji and New Caledonia one each) and Paragnathiella (1, Canary Islands).
CLAUSOGNATHIIDAE
A single species disjunct from Belize and Panama.
GNATHOSTOMARIIDAE
A single spp. from Mediterranean region and North Carolina in USA.
GNATHOSTOMULIDAE
(5/)24 spp. in Chirognathia (1, Vancouver region and California), Corculognathia (1, Galapagos), Gnathostomula (19, NW Atlantic, Fiji, NE Pacific, Galapagos, Bahamas, Caribbean, W Europe, Adriatic, Barents, Red Sea, Maldives, Tahiti, New Zealand one each), Ratugnathia (1, Fiji) and Semaeognathia (1, North Carolina and Florida Keys, USA). One undescribed species in Brazil in Gnathostomula — NE Pacific (2/2), Galapagos (1/1), NW Atlantic (1/1), Bahamas (1/1), SE USA (1/1), Caribbean (1/1), W Europe (1/1), Adriatic (1/1), Barents (1/1), Red Sea (1/1), Maldives (1/1), Tahiti (1/1), New Zealand (1/1), Fiji (1/1).
MESOGNATHARIIDAE
(3/)6 spp. in Labidognathia (1, Canary Islands, Caribbean, Australia), Mesognatharia (3, Sweden, Bahamas, Georgia and North Carolina, USA) and Tenuignathia (2, one in Florida, North Carolina, Bermuda, and one in Fiji) — Canary Islands (1/1), Bahamas (1/1), Caribbean (1/1), SE USA (2/2), Sweden (1/1), Australia (1/1), Fiji (1/1).
ONICHOGNATHIIDAE
(5/)9 spp. in Goannagnathia (1, N Papua New Guinea to NW Australia until Brisbane), Nanognathia (1, North Carolina and Florida Keys, USA), Onychognathia (3, North Carolina and Florida Keys, USA, Belize, Panama, Galapagos), Valvognathia (1, Zealand, Denmark) and Vampyrognathia (3, North Carolina and Florida Keys, USA, one each one in NW Australia) — Galapagos (1/1), SE USA (3/3), Belize (1/1), Panama (1/1), Denmark (1/1), Papua New Guinea (1/1), Australia (2/2)..
PAUCIDENTULIDAE
A single species from Belize.
PROBLONGNATHIIDAE
A single species from Bermuda.
RASTROGNATHIIDAE
A single species from Denmark.
▉ endemic families in New World: Paucidentulidae (1/1, Belize), Problognathiidae (1/1, Bermuda).
▉ LAST UPDATE OF GNATHOSTOMULIDA IN JANUARY/FEBRUARY 2026
8. MICROGNATHOZOA ‣ two species have been reported in this phyllum: Limnognathia maerski Kristensen & Funch (Wikipedia), discovered in 1994 on Disko Island, Greenland, although not formally described until 2000, and later observed in several other locations: S Wales, UK; River Lambourn in Berkshire, UK (Bekkouche et al., Frontiers in Zoology, 2014); and Bassa Nera pond in the Pyrenees of NE Spain (Giribet et al., Current Biology, 2023); and L. desmeti Worsaae & Møller, 2025, found in the Crozet Archipelago, Île de la Possession, at Pointe du Bougainville (Sayo et al., Proceedings of the Royal Society, 2025).
Additionally, one species was detected in SE Brazil through eDNA metabarcoding of samples collected in 2018 from the Rio Doce estuary, Espírito Santo state. Although this record has not yet been formally published or included in national checklists, it is already considered valid (Coppo et al., Peerj, 2023).
▉ LAST UPDATE OF MICROGNATHOZOA IN JANUARY/FEBRUARY 2026
9. SYNDERMATA ‣ apart from the traditional classification that separates Rotifera and Acanthocephala into two distinct phyla (even in Catalogue of Life/Phyla), here we treat them as a single entity, Syndermata, following modern studies such as Laumer et al. (Proceedings of the Royal Society, 2019) and Giribet et al. (BOOK, 2023). Syndermata comprises seven primary lineages, all designated at the class level. According to this work, 69 monogononts, one bdelloid, and all Seisonida species are exclusively marine, while the remaining species inhabit freshwater or brackish environments (with 182 spp. occurring in marine settings — Frontier Sin/2023). Segers (Zootaxa, 2007) lists 2,030 Rotifera worldwide (3 in Seisonida, 1,570 in Monogononta, 461 in Bdelloidea).
(68:336/)4,174 spp. worldwide, (47:125/)687 in Brazil, numbers based on a compilation of the data below analyzed by class.
All data presented below follow Catalogue of Life (Catalogue of Life/Rotifera, Catalogue of Life/Acanthocephala) at the global level and CTFB (CTFB/Rotifera, CTFB/Acanthocephala) for Brazil, with additions of some species, genera and families at Brazilian count.
Mexico alone hosts (27:75/)402 spp. of Eurotatoria (Sarma, MDPI, 2021). Other checklist worldwide includes freshwater Rotifers from Thailand (409 spp., Jaturapruek & Maiphae, Biodiversity Data Journal, 2025).
PARAROTATORIA
8 spp. in Seison (3) and Paraseison (5) worldwide, parasiting Nebalia (Malacostraca/Leptostraca), known from Adriatic Sea, Tyrrhenian Sea, Balearic Archipelago and along the Atlantic coast of France, Sea of Okhotsk, NW Pacific (but the identity of this species is questionable), Gazi Bay in Kenya, W USA, and unidentified specimens from S Chile (Francesca Leasi et al., JMBAUK, 2012).
MONOGONONTA
(32:134/)2,193 spp. worldwide, and (27:78/)569 in Brazil. Five families does not occur in Brazil: Asciaporrectidae, Birgeidae (1/1, endemic to E North America, Balian, 2008), Clariaidae (1/1, Vietnam, Systematics Rotifera | Rotifera Hausdernatur), Cotylegaleatidae (1/2, Belgium and Turkey one endemic each, see De Smet & Bozkurt, Zootaxa, 2016), and Microcodonidae, all in order Ploima.
BDELLOIDEA
(5:22/)488 spp. worldwide (Catalogue of Life), and (3:8/)38 in Brazil. Two families does not occur in Brazil: Coronistomidae (endemic to USA, Örstan, Zootaxa, 2021), and Philodinavidae (New Zealand, Europe, North America, Sumatra, South Africa, South America, Hawaii, Ricci & Melone, Hydrobiologia, 1998).
ARCHIACANTHOCEPHALA
(4:22/)187 spp. worldwide, (4:8/)25 in Brazil (CTFB | Apororhynchus aculeatus Meyer, 1931 | Gigantorhynchus echinodiscus (Diesing, 1851) | Neoncicola-5). Some American Latina genera do not occur in Brazil (Amin, UNAM, 2000): Apororhynchus, Gigantorhynchus (Colombia, Peru, Venezuela) and Neoncicola (Puerto Rico, Venezuela).
EOACANTHOCEPHALA
(4:33/)319 spp. worldwide, (2:9/)21 in Brazil (CTFB | Pandosentis iracundus). Some American Latina genera do not occur in Brazil (Amin, UNAM, 2000): Acanthogyrus (Puerto Rico, T.Tobago), Deltacanthus (Venezuela) and Wolffhugelia (Uruguay).
PALAEACANTHOCEPHALA
(21:122/)975 spp. worldwide, (10:21/)32 in Brazil (CTFB | Heterosentis brasiliensis Vieira, Felizardo & Luque, 2009, Arythmacanthidae | Serrasentis sp., Isthmosacanthidae). Some American Latina genera do not occur in Brazil (Amin, UNAM, 2000): Breizacanthus (Argentina, FP), Neoacanthocephaloides (Puerto Rico), Caballerorhynchus (Mexico), Megapriapus (Venezuela), Pseudocavisoma (Puerto Rico), Hypoechinorhynchus (Argentina), Tegorhynchus (Juan Fernandez, Puerto Rico), Pomphorhynchus (Argentina, Chile, Mexico), Pseudoleptorhynchoides (Mexico) and Plagiorhynchus (Mexico).
POLYACANTHOCEPHALA
(1:1/)4 spp. worldwide, (1:1/)2 in Brazil.
▉ endemic families in New World: Coronistomidae (1/1, USA).
▉ LAST UPDATE OF SYNDERMATA IN JANUARY/FEBRUARY 2026
PLATYTROCHOZOA
10. ORTHONECTIDA ‣ (2:5/)24 spp. of parasites of marine invertebrates, mainly in Mollusca, Platyhelminthes, Acoelomorpha and Annelida, collected at their hosts in Atlantic coast of Europe, Arctic, W North America and Japan (Wikipedia), all in northern Hemisphere. Maximum likelihood analyses placed the Dicyemida + Orthonectida clade within the Gastrotricha, while in Bayesian inference analyses, this clade is sister group to the clade of Gastrotricha + Platyhelminthes (Tsai-Ming Lu et al., Zoological Letters, 2017).
RHOPALURIDAE
(4/)23 spp. (Kozloff, CBM, 1992 | Kozloff, CBM, 1993 | Slyusarev & Manilov (CBM, 2021) in Stoecharthrum (4, NW France-2 and Pacific coast of NW USA-2), Ciliocincta (3, NW France-1, NW USA-1 and Hokkaido in Japan-1), Intoshia (6, NW France-4, Scandinavia-1, Kola Peninsula in NW Russia-1) and Rhopalura (10, W USA to Washington to California, Sweden, SW Italy, NW France, SW England, Murmansk coast of Russia).
PELMATOSPHAERIDAE
A single genus and species, Pelmatosphaera polycirri Caullery and Mesnil, 1904 from NW France and UK, collected in Annelida and Nemertea (SE Ferriss et al., Irish Biodiversity, 2009).
▉ LAST UPDATE OF ORTHONECTIDA IN JANUARY/FEBRUARY 2026
11. DICYEMIDA ‣ (3:9/)147 spp. (Catalogue of Life/Dicyemida) of tiny parasites that inhabit the renal appendages of Cephalopoda (Wikipedia), never recorded along the Brazilian coast. Maximum likelihood analyses placed the Dicyemida + Orthonectida clade within the Gastrotricha, while in Bayesian inference analyses, this clade is sister group to the clade of Gastrotricha + Platyhelminthes (Tsai-Ming Lu et al., Zoological Letters, 2017).
CONOCYEMIDAE
(2/)2 spp., Conocyema polymorpha Van Beneden, 1882, from Atlantic coast of France, UK, Italy and Monaco (SE Ferriss et al., Irish Biodiversity, 2009 | Furuya & Souidenne, HPDC, 2019), and Microcyema vespa Van Beneden, 1882
from Plymouth, UK, Roscoff in France, and in Monaco (Furuya & Hochberg, Life & Environment, 1999).
DICYEMIDAE
Widely worldwide, (6/)144 spp. in six genera: Dicyema (80), Dicyemmenea (50), Dicyemodeca (4), Dodecadicyema (1, E India), Pleodicyema (8) and Pseudicyemma (8, 5 in Japan), known from E Canada (1/1), USA (2/21), Mexico (1/11, SP, 2016, 8 in Pacific, 3 in Caribbean), Venezuela (1, Penchaszadeh et al., Journal of Molluscan Studies, 1996, genus unknown), Argentina (2/4 — JIP, 2026), Mauritania (1/2), Spain (1/1), France (1/4), Sweden (1/1), Norway (1/1), Italy (2/4), W Mediterranean (2/6), India (3/6), Japan (4/56 — 14 new, 2022), Russia (3/7), New Zealand (2/4), Subantarctic Islands (1/4), International waters (3/5), Antarctica (1/1) and Australia (2/10, SEE | SEE | SEE), mainly listed in Catalano, S. (Zootaxa, 2012).
KANTHARELLIDAE
A single species known only from Weddel Sea, Antarctica.
▉ LAST UPDATE OF DICYEMIDA IN JANUARY/FEBRUARY 2026
12. GASTROTRICHA ‣ (20:118/)932 spp. worldwide, in both marine and freshwater environments, (10:30/)89 in Brazil. Minowa & Garraffoni (Zoologia, 2025) cites 13 spp. of freshwater Gastrotricha in Brazil. All data presented below follow Catalogue of Life/Gastrotricha at the global level and CTFB/Gastrotricha for Brazil.
CHAETONOTIDA
(8:40/)539 spp. worldwide, (4:21/)76 spp. in Brazil. 2/3 of the species of Chaetonotida are found in continental waters, but Muselliferidae and Xenotrichulidae are exclusively marine (Campos and Garraffoni, PeerJ, 2019).
MACRODASYIDA
(12:38/)393 spp. worldwide, all marine except four reported in freshwater: Marinellina flagellata Ruttner-Kolisko, 1955 (Austrian river Ybbs), Redudasys fornerisae Kisielewski, 1987 (Brazilian dam on the savannah near São Carlos city), and two in streams and aquifer in USA (Garraffoni et al., ZooKeys, 2010). (6:9/)13 spp. in Brazil.
▉ endemic families in New World: Hummondasyidae (1/1, Macrodasyda, Jamaica).
▉ LAST UPDATE OF GASTROTRICHA IN JANUARY/FEBRUARY 2026
13. PLATYHELMINTHES ‣ simple bilaterian, unsegmented, soft-bodied worms commonly called flatworms. (514:5,163/)28,638 spp. worldwide and (216:939/)2,398 in Brazil, numbers based on a compilation of the data below analyzed by the two subphylla (Rhabditophora and Catenulida) and the taxa Incertae sedis (38 unplaced genera, unknown number of species), mainly Catalogue of Life for world and CTFB/Platyhelminthes in Brazil, except by small changes in Cavernicola, Maricola, Gnosonesimida, and position of Duplominona and Urastomidae.
The massively polyphyletic group ‘Turbellaria’ was split into several lineages, and the parasitic groups Trematoda, Monogenea, and Cestoda were combined into the new clade Neodermata. To organize Platyhelminthes into monophyletic groups while maintaining Neodermata as one of them, we structured nine groups in a hierarchical framework based on the phylogeny implicitly suggested by the Turbellarian Taxonomic Database (TTD), accessed on December 16, 2024. In this classification, (1) Catenulida appears as the basal lineage, followed by (2) Macrostomorpha. Trepaxonemata is divided into three groups: (3) Amplimatricata, (4) Gnosonesimida, and Euneoophora. The latter splits into (5) Rhabdocoela and a clade comprising (6) Proseriata and Acentrosomata (Wikipedia). This clade, in turn, divides into (7) Adiaphanida, (8) Bothrioplanida, and (9) Neodermata. Bolded names represent the accepted canonical lineages.
A total of 13,214 species occur in marine environments (Frontier Sin/2023).
For Brazil, detailed cheklists outdated includes Braccini, J.A.L., Amaral, S.V. and Leal-Zanchet, A.M. (Braz. J. Biol., 2016) for numbers for Catenulida, Macrostomorpha, Rhabdocoela, Proseriata, Prolecithophora and Fecampida in Adiaphanida, Prorhynchida in Amplimatricata and Bothrioplanida; Carbayo et al. (Biota Neotropica, 2008) provides numbers for Polycladida in Amplimatricata and Tricladida in Adiaphanida. Together, these works lists 420 spp. in Brazil.
Some checklist worldwide includes Actoylea from Caribbean Colombia (10:14/22 spp., Merchán-Mayorga, JI et al., Taxonomy, 2025) and free-living flatworms in Cuba (279 spp., Díez, YL et al., Biological Journal of the Linnean Society, 2023).
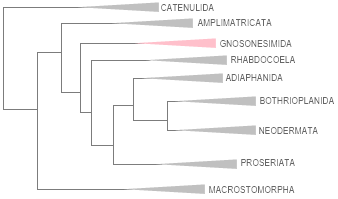
PHYLOGENY OF PLATYHELMINTHES AFTER TTD ACCEPTED HERE
INCERTAE SEDIS
Apart this two large groups, 38 taxa (genera, small clades, mainly monotypics) are incertae sedis, unplaced among any clade, with one known for Brazil, Candimba divae Marcus, 1949.
A SUBPHYLLUM RHABDITOPHORA
(508:5,113/)28,525 spp. worldwide and (213:932/)2,351 in Brazil, numbers based on a compilation of the data below analyzed by the three lineages (Trepaxonemata and Macrostomatida).
A1 RHABDITOPHORA/TREPAXONEMATA
(504:5,083/)28,217 spp. worldwide and (210:926/)2,335 in Brazil, numbers based on a compilation of the data below analyzed by the three lineages (Amplimatricata, Euneoophora, Gnosonesimida).
AMPLIMATRICATA
(53:223/)1,127 spp. worldwide and (19:41/)58 spp. in Brazil.
Polycladida ‣ (52:219/)1,097 spp. worldwide, (18:39/)56 in Brazil, 7 endemic genera. Excludes Duplominona.
[ENDEMIC FAMILIES IN BRAZIL, ECUADOR, ARGENTINA].
Prorhynchida (1:4/30 spp. in Prorhynchidae, Geocentrophora applanata Kennel, 1888 and Prorhynchus stagnalis Schultze, 1851 in Brazil).
[TROGLOBIC IN USA - 1/1].
EUNEOOPHORA
(450:4,859/)27,090 spp. worldwide and (191:885/)2,277 in Brazil, numbers based on a compilation of the data below analyzed by the three lineages (Acentrosomata, Proseriata, Rhabdocoela).
ACENTROSOMATA
(400:4,333/)24,666 spp. worldwide and (171:831/)2,181 in Brazil, numbers based on a compilation of the data below analyzed by the five lineages.
ADIAPHANIDA/ORDER TRICLADIDA
(13:184/)1,924 spp. worldwide, (6:36/)240 spp. in Brazil (excludes Urastomidae, placed here in Fecampiida). Tricladida has three lineages: Cavernicola, Continenticola and Maricola.
[TROGLOBIC WORLDWIDE].
CONTINENTICOLA
(5:135/)1,821 spp. in Dendrocoelidae (24/259), Kenkiidae (3/24), Planariidae (12/185), Dugesiidae (12/235) and Geoplanidae (77/993). In Brazil (28/)232 spp. in two families: Dugesiidae (2/21 in Bopsula-1 and Girardia-20) and Geoplanidae (26/211, 73 in Geoplana). All Brazilian obligatory subterranean Platyhelminthes belongs order Tricladida [1] in all three last clades: Continenticola (10 in Girardia, Dugesiidae), Maricola (1) and Cavernicola (2). Sluysia triapertura Leal-Zanchet & Souza, 2018 represents the first Maricola triclad living in freshwater within a cave. Brazil is the only country in the world with troglobitic representatives in the three groups of Tricladida (SEE). Dimarcusidae is a clade known only from Brazil, Mexico and America Central, Nigeria and Borneo (L. Benítez-Álvarez, et al., Molecular Phylogenetics and Evolution, 2020).
CAVERNICOLA
(2:7/)10 spp. worldwide (plus one undescribed Hausera in Brazil). (1:2/)3 spp. in Brazil.
MARICOLA
(6:42/)93 spp. worldwide. Brazil has (6/)6 spp., in Cercyridae (1/1), Procerodidae (1/1) and Uteriporidae (4/4, including Slyusia and Tiddles).
ADIAPHANIDA/ORDER PROLECITOPHORA
(7:36/)201 spp. worldwide, (3:11/)26 in Brazil in Plagiostomidae (6/18), Pseudostomidae (4/7, including Cylindrostomidae) and Scleraulophoridae (1/1).
ADIAPHANIDA/ORDER FECAMPIIDA
(5:8/)18 spp. worldwide (includes Genostomatidae, sometimes placed outside Fecampiida), Urastoma cyprinae Graff, 1882 (Urastomidae) in Brazil.
BOTHRIONEODERMATA/ORDER BOTHRIOPLANIDA
(2/)4 spp. in a single family, one in Brazil (Bothrioplana semperi Braun).
BOTHRIONEODERMATA/NEODERMATA
(374:4,103/)22,519 spp. worldwide, all parasites, in three groups: Cestoda (105:1,094/3,777), Monogenea (64:809/6,125) and Trematoda (205:2,200/12,617). Brazil has (38:166/)391 spp. of Cestoda, (29:174/)665 spp. in Monogenea and (93:440/)857 in Trematoda (Aspidogastrea and Digenea), totalizing (160:780/)1,913 of Neodermata.
[TROGLOBIC IN USA - 2/2].
[ENDEMIC FAMILIES IN USA, BRAZIL, PERU]
PROSERIATA
(13:113/)532 spp. worldwide, four unplaced genera at family level. (4:14/)19 spp. in Brazil, in Coelogynoporidae (1/1), Monocelididae (6/9, includes Duplominona), Nematoplanidae (3/4) and Otoplanidae (4/5).
[TROGLOBIC IN SOUTH AFRICA - 1/1].
RHABDOCOELA
(37:413/)1,892 spp. in three clades: Kalyptorhynchia with (18:178/)716 spp. worldwide, with one unplaced genus and (6:14/)17 spp. in Brazil; Dalytyphloplanida with (18:232/)1,172 spp. worldwide, with four unplaced genera, (10:24/)60 spp. in Brazil, including Temnocephalidae and Dalyelliidae; and Mariplanellida with (1:3/)4 spp., known from northern Europe, Kerguelan Is. and Curazao (Marine Species). Over, (16:40/)77 spp. occur in Brazil.
[TROGLOBIC IN EUROPE - 1/13].
[ENDEMIC FAMILY IN USA].
GNOSONESIMIDA
(1/)7 spp. in Gnosonesima, known from Massachusetts (USA), Antarctica (G. antarctica), North Sea and Greenland (G. borealis and G. brattstroemi), Mediterranean Sea (G. mediterranea) and Somalia (G. tropicalis), by PlanMine (SEE), and unnamed records in California (GBIF), Panama (Panamabiota) and SE Cuba (Diez et al., BJLS, 2023).
A2 RHABDITOPHORA/MACROSTOMORPHA
(4:33/)308 spp. worldwide in four lineages, (3:6/)16 spp. in Brazil.
Bradynectes ‣ (1/)6 spp., not recorded in Brazil.
Dolichomicrostomida ‣ (2:19/)89 spp. in two families, Dolichomacrostomidae (13/38, 2/2 in Brazil in Austromacrostomum and Karlingia) and Microstomidae (6/51, 2/7 in Brazil, in Macrostomum-6 and Nerpa-1). Over, (2:3/)8 spp. in Brazil.
Haplopharyngidae ‣ (1/)3 spp. from coasts of Belgium and Norway (Haplopharynx rostratus Meixner, 193), southern France (H. quadristimulus Ax, 1971) and Italy and Portugal (H. papii Schockaert, 2014), by Schockaert (Zootaxa, 2014), plus unverified records in North Carolina (USA) and Panama.
Macrostomidae ‣ (12/)210 spp., 2/7 in Brazil, in Macrostomum-6 and Myozona-1.
B SUBPHYLLUM CATENULIDA
(6:12/)113 spp. worldwide, (3:7/)46 in Brazil, in Catenulidae (3/9), Chordariidae (1/4) and Stenostomidae (4/33).
▉ endemic families in New World, all Rhabditophora: Atamatamidae (3/3, Neodermata, Peru), Mucroplanidae (1/1, Amplimatricata, Ecuador), Discoprosthididae (1/1, Amplimatricata, Argentina), Euryleptididae (1/1, Amplimatricata, Brazil), Braunotrematidae (1/1, Neodermata, Brazil), Crassicollidae (1/1, Rhabdocoela, USA) and Acipensericolidae (1/2, Neodermata, USA).
▉ LAST UPDATE OF PLATYHELMINTHES IN JANUARY/FEBRUARY 2026
14. ENTOPROCTA ‣ (4:15/)171 spp. worldwide (Catalogue of Life/Entoprocta | it is worth noting that the Catalogue of Life makes a serious mistake by swapping the names of the two orders that make up this phylum.) in two orders, (4:6/)17 spp. in Brazil (CTFB/Entoprocta).
Only two species of Entoprocta lives in freshwater, both in Coloniales: Loxosomatoides sirindhornae Wood, 2005 (Wood, Hydrobiologia, 2005), reported in 2004 in C Thailand, and Urnatella gracilis Leidy, 1851, found in all the continents except Antarctica (Wikipedia), also in Brazil, both in Urnatellidae.
SOLITARIA
(6/)152 spp. in a single family worldwide, Loxosomatidae. (1/)7 spp. in Brazil.
COLONIALES
Here we follow Borisanova & Ekimova (Invertebrate Zoology, 2025), who proposes the family Urnatellidae for Barentsia benedeni (Foettinger, 1887) and Urnatella gracilis Leidy, 1852, removing them from Barentsiidae, and transfers Loxosomatoides (4) from Pedicellinidae (including L. sirindhornae Wood, 2005, a freshwater species endemic to Thailand). As a result, the two freshwater species of Entoprocta are placed in the same family, Urnatellidae (3/6).
(12/)52 spp. in four families: Barentsiidae (4/28), Pedicellinidae (5/16), Loxokalypodidae (1/2) and Urnatellidae (3/6), (3:5/)10 in Brazil. Loxokalypodidae, unique family unknown in Brazil, has (1/)2 spp., one from Queen Charlotte Islands, Canada, in northern Pacific (Wasson, ZJLS, 1997), and one from S New Caledonia (WORMS).
▉ LAST UPDATE OF ENTOPROCTA IN JANUARY/FEBRUARY 2026
15. CYCLIOPHORA ‣ a phylum consisting of two described (Catalogue of Life) and one undescribed microscopic specieswithin a single genus Symbion and family Symbiidae, parasitizing lobster gills in the northern Atlantic, and showing strong affinities with Entoprocta and Ectoprocta.
S. americanus Obst, Funch and Kristensen, 2006 ‣ parasite in Homarus americanus H. Milne-Edwards, 1837, collected in Halifax (Canada), Maine, Nova York, Massachussets and Maryland in NE USA (Obst et al., Molecular Ecology, 2005), at three distinct lineages, possibly three species (Sato et al., Invertebrate Biology, 2022).
S. pandora Funch and Kristensen 1995 ‣ parasite in Nephrops norvegicus L., 1758, collected in coasts of Sweden, Denmark, Faroe Is., UK, France, Spain and Croatia (Obst et al., Molecular Ecology, 2005).
Symbion sp. ‣ parasite in Homarus gammarus L., 1758 (Wikipedia), collected on Norway, Denmark, France and Croatia (Obst et al., Molecular Ecology, 2005), possibly infecting, in some life stage, harpacticoid copepods in coastal France (Neves & Xavier, Organisms, Diversity & Evolution, 2014).
▉ LAST UPDATE OF CYCLIOPHORA IN JANUARY/FEBRUARY 2026
16. NEMERTEA ‣ unsegmented, strongly elongated, slender, soft, and usually without appendages worms. (49:319/)1,427 spp. worldwide in four classes (Catalogue of Life). (12:19/)39 spp. in Brazil (CTFB/Nemertea).
Only four spp. of Nemertea are freshwater in New World: Prostoma eilhardi Montgomery, 1894 (Argentina and Brazil), P. graecense Böhmig, 1892 (Venezuela), Koinoporus mapochi Sanchez & Moretto, 1988 (known from small streams flowing into the Mapocho River in Chile), and Siolineus turbidus Du Bois-Reymond Marcus, 1948 (known from four females in the Brazilian river Tapajós, found at 28 m depth in the 1940s). The latter is the only Neotropical freshwater species representing Pilidiophora/Heteronemertea (KNAF/2020).
ARHYNCHOCOELA
A single species, Arhynchonemertes axi Riser, 1988, unique Nemertea without proboscis and rhynchocoel, restricted to New Zealand (Chernyshev, Hydrobiological Journal, 1985 | Chernyshev, , Invertzool, 2021).
PALAEONEMERTEA
(6:11//)121 spp. worldwide (Catalogue of Life), (2:2/)2 spp. in Brazil, formerly Anopla.
PILIDIOPHORA
(7:116/)534 spp. worldwide in a single order Heteronemertea (Catalogue of Life), (2:5/)7 in Brazil, also formerly Anopla.
HOPLONEMERTEA
(35:191/)771 spp. worldwide, (8:12/)30 in Brazil. Currently, about (7/)14 spp. grouped in seven genera are known inhabiting fully terrestrial or intermediate habitats, in two families, both of Hoplonemertea (Moore & Gibson, Biological Reviews, 1985): Acteonemertidae (Acteonemertes-1 and Antiponemertes-3 in New Zealand, Argonemertes-4 in Australia, Katechonemertes-1 in Tristan da Cunha, and Leptonemertes-1, scattered records in antropic zones in Europe, California and Canary Islands) and Prosorhochmidae (Geonemertes-2, widespread on Indo-Pacific islands from Mauritius and Rodriguez to Samoa and Palau across Philippines and New Guinea, and has also been found in Dominica, Caribbean and Florida; and Pantinonemertes-2, in Australia-1 and Bermuda-1).
▉ endemic families in New World: Pachynemertidae (1/1, Enopla, Bermuda).
▉ LAST UPDATE OF NEMERTEA IN JANUARY/FEBRUARY 2026
17. MOLLUSCA ▸ (816:10,051/)98,119 spp. worldwide, in marine, freshwater and terrestrial environments, numbers based on a compilation of the data below analyzed by order, the second most speciose invertebrate phyllum after Arthropoda (Wikipedia). Mollusca phylogeny accepted here is based on Xiaolu Han et al. (Front. Ecol. Evol., 2024), with two high clades: Aculifera (Polyplacophora + [Caudofoveata + Solenogastres]) and Conchifera ([Monoplacophora + Cephalopoda] + [Bvalvia + [Gastropoda + Scaphopoda]]), in eight living classes.
(401:1,358/)3,561 spp. in Brazil: Caudofoveata (3:5/10 — CTFB), Solenogastres (3:7/12 — CTFB | Cobo & Chiongco, Marine Biodiversity, 2025), Polyplacophora (7:11/37 — CTFB), Scaphopoda (6:19/42 — CTFB), Cephalopoda (37:79/94 — CTFB), Bivalvia (80:305/629 — F.M. Machado et al., Zoologia, 2023) and Gastropoda (265:932/2,737 — F.M. Machado et al., Zoologia, 2023).
In freshwater environments, Brazil harbors (7:29/)116 species of Bivalvia and (10:33/)177 spp. species of Gastropoda (F.M. Machado et al., Zoologia, 2023). In terrestrial environments, Brazil has (35:126/)715 species of Gastropoda (Salvador, RB et al., Journal of Conchology, 2024). All remaining species are marine. Apparently, no family of Gastropoda or Bivalvia occurs in more than one of these three habitat types. South America includes at least 1,401 non-marine mollusca (Miyahira et al., Biodiversity and Conservation, 2022).
The deepest record of Mollusca recorded in Brazil belongs Bentharca asperula (Dall, 1881), off Rio de Janeiro and São Paulo states (F.M. Machado et al., Zoologia, 2023 | Passos & Birman, Biota Neotropica, 2009).
Mexico harbors 4,643 marine species of Mollusca (Castillo-Rodríguez, Revista Mexicana de Biodiversidad, 2014), comprising 3,127 Gastropoda—1,712 in the Pacific and 1,415 in the Atlantic — 1,202 Bivalvia, 159 Polyplacophora, 111 Cephalopoda, 40 Scaphopoda, 3 Monoplacophora, and a single Aplacophora. In freshwater environments, Mexico contains 193 species of Gastropoda and 97 species of Bivalvia (Czaja, Revista Mexicana de Biodiversidad, 2020). Terrestrial environments host 1,184 species of Gastropoda (Naranjo-García, Revista Mexicana de Biodiversidad, vol. 85, vol. 85, 2014).
Other national checklist includes land and freshwater Mollusca from Portugal (195 spp., Holyoak, D et al., Iberus, 2019), annotated checklist of marine mollusca from Madeira and Selvagens archipelagos from Portugal (850 spp., Willy, S. et al, Bocagiana, 2009), and marine Mollusca from Spain jurisdiction (2,466 spp., Gofas, S et al, Scientia Marina, 2017).
Currently, 41 aquatic non-native mollusk species have been identified in South America, comprising 24 marine species (10 gastropods and 14 bivalves) and 17 freshwater species (12 gastropods and 5 bivalves). Among these, the freshwater clam Corbicula fluminea Müller,1774, the golden mussel Limnoperna fortunei Dunker, 1857, and the Pacific oyster Magallana gigas Thunberg, 1793 stand out for their significant impacts on native species and ecosystems (Darrigran et al., Biology, 2025).
POLYPLACOPHORA
(21:142/)1,134 spp. worldwide (Catalogue of Life), (7:11/)37 in Brazil (CTFB/Polyplacophora).
CAUDOFOVEATA
(3:14/)144 spp. worldwide (Catalogue of Life), (5/)10 in Brazil in all families (CTFB/Caudofoveata).
SOLENOGASTRES
(25:94/)330 spp. worldwide (Catalogue of Life), (3:7/)12 in Brazil (CTFB/Solenogastres | Cobo & Chiongco, Marine Biodiversity, 2025).
CEPHALOPODA
(54:226/)987 exclusive marine spp. worldwide, numbers based on a compilation of the data below analyzed by order, (37:79/)94 in Brazil (CTFB/Cephalopoda), divided in two high clades. All clades occur in Brazilin coasts except Nautiloideae and Idiosepida.
Nautiloideae ‣ (2/)11 spp. among a single family, from Myanmar to NW Australia, and from Philippines to Samoa (MAP/1 | MAP/2).
Neocoleoidea ‣ eight living orders and (224/)976 spp. in 53 families, 7 orders in Brazil:
SPIRULIDA
A single species, Spirula spirula L., known from mainly tropical and temperate areas in world, including Brazil.
SEPIIDA
Including Sepiolida sensu Catalogue of Life. (43/)212 spp., in three living families: Sepiadariidae (2/7, bottletails, India to New Zealand, northern up to Japan, and some islands off Chile), Sepiidae (3/110, cuttlefishes, not recorded in New World waters) and Sepiolidae (15/62 spp. worldwide, 5/6 in Brazil).
IDIOSEPIDA
A single family, Idiosepiidae, (4/)12 spp. from southern Africa to Japan, southern up to NE Australia (FAO, 2006).
MYOPSIDA (sometimes as Teutida)
Coastal squid, (13/)51 spp. two families: Australiteuthidae (1/1, NW Australia and New Guinea, Wikipedia), and Loliginidae (5/45) widely worldwide (Wikipedia), with (4/)6 spp. in Brazil in latter family.
OEGOPSIDA
Neritic squids, (26:92/)317 spp. worldwide, (19:40/)47 spp. in Brazil.
BATHYTEUTHIDA
(2:2/)10 spp. worldwide in Bathyteuthidae and Chtenopterygidae, (2:2/)2 in Brazil, in both families.
OCTOPODA
(68/)371 spp. in 17 families, 12 in Brazil (26/31, Cirroteuthidae, Opisthoteuthidae, Stauroteuthidae, Alloposidae, Argonautidae, Ocythoidae, Tremoctopodidae, Amphitretidae, Eledonidae, Enteroctopodidae, Megaeledonidae, and Octopodidae), and 5 not recorded in country: Cirroctopodidae (1/4, southern cold waters), Idiooctopodidae (1/1), Bathypolypodidae (1/8), Laetmoteuthidae (1/2) and Grimpoteuthididae (2/13).
VAMPYROMORPHIDA
Two spp., Vampyroteuthis infernalis Chun, 1903, a small cephalopod found throughout temperate and tropical oceans in extreme deep sea conditions, including Brazil, and V. pseudoinfernalis D.J. Qiu, B.L. Liu & L.M. Huang, 2024 (coast of China, Zoological Systematics).
MONOPLACOPHORA
A relictual group not recorded in Brazil. (4:7/)31 spp. worldwide (Waren & Gofas, Zoologica Scripta, 2005) | Marshall, Molluscan Research, 2006) | D. L. Ivanov & Moskalev, Ruthenica, 2007) | Y. Kano et al., Zoologica Scripta, 2012) | Schwabe, Zootaxa, 2008), two families in New World. Highest diversities in New Zealand (2:2/6), Mexico (2:4/4), Azores region (1:1/4) and Peru (1:1/4).
LAEVIPILINIDAE ‣ 5 spp. i a single genus, Laevipilina antarctica, L. theresae (these in Antarctica), L. cachuchensis, L. rolani (these off N Spain), and L. hyalina (Washington to NW Baja California, Mexico).
MICROPILINIDAE ‣ 5 ssp. in a single genus: Micropilina arntzi (Antarctica), M. minuta (off Iceland and Italy), M. rakiura, M. reingi, M. tangaroa and M. wareni (all in NW New Zealand).
MONOPLACOPHORIDAE ‣ a singles species, Monoplacophorus zenkevitchi Moskalev, Starobogatov & Filatova, 1983, from center Pacific, north of Johnston Islands, west of Hawaii.
NEOPILINIDAE ‣ 4/19 spp., in Adenopilina (1, Gulf of Aden), Neopilina (Peru/Chile-1, Mexico to Galapagos-1, Malvinas/Argentina-1, E Kamchatka/Russia-1), Veleropilina (Mid-Atlantic Ridge-2, Chatham Rise/New Zealand-1, Virgin Islands/Caribbean- 1, N Hawaii-1, Corsica/Sardenha/S Italy-1, SE Honshu island/Japan-1, W Baja California/Mexico-1, NE Azores-1), Vema (Peru-Chile zone-3, SW Mexico-1, NW New Zealand-1).
BIVALVIA
(124:1,668/)10,858 spp. worldwide, 1,371 of these in freshwater (Molluscabase), (80:305/)629 in Brazil (55:209/527 marine and 8:29/116 freshwaters), numbers based on a compilation of the data below analyzed by clade. Two high clades worldwide.
One the best works for freshwater bivalves is Graf (Amer. Mallac. Bull., 2013), that shows 1,178 spp. worldwide. Neartic region includes only three native families of freshwater bivalves, with 343 spp., the largest amount by region, 295 in Unionidae. Neotropical region includes (8:)249 spp., the 3rd amount by region. Gulf of Mexico includes (75:314/)640 spp. of Bivalvia in 17 orders (Mozo et al., Bulletin of Marine Science, 2024).
SUBCLASS AUTOBRANCHIA
(110:1,586/)9,980 spp. worldwide, (51:215/)589 in Brazil, numbers based on a compilation of the data below analyzed by clade. Two clades worldwide, both with freshwater members, only one in South America.
INFRACLASS HETEROCONCHIA
(80:1,292/)7,667 spp. worldwide, (51:215/)449 in Brazil, numbers based on a compilation of the data below analyzed by clade. Three clades worldwide, two with freshwater members, both in South America.
SUBTERCLASS ARCHIHETERODONTA
(4:76/)431 spp. worldwide (Catalogue of Life), (2:8/)12 in Brazil (CTFB/Bivalvia| Machado, FM et al., Zoologia, 2023).
SUBTERCLASS EUHETERODONTA
(69:979/)6,060 spp. worldwide (Catalogue of Life), (46:175/)349 in Brazil (CTFB/Bivalvia). Includes 270 freshwater species in 20 genera, only 7 in New World: Neocorbicula (4, Cyrenidae), Mytilopsis (2, Dreissenidae), Anticorbula (1, Corbulidae), Eupera, Byassanodonta, Pisidium and Sphaerium (these in Sphaeridae).
Grippina coronata Machado & Passos, 2015 (Euheterodonta, Spheniopsidae) endemic to E coast of Brazil, is the smallest carnivorous bivalve known, with c. 1mm diameter (Morton, B. et al., Journal of Molluscan Studies, 2015).
SUBTERCLASS PALAEOHETERODONTA
(7:237/)1,176 spp. worldwide (Catalogue of Life), (2:18/)84 in Brazil (CTFB/Bivalvia | Machado, FM et al., Zoologia, 2023). One family exclusively marine (Trigoniidae) and six exclusively freshwater: Unionidae (681, North and America Central, Eurasia, Africa), Margaritiferidae (13, Holarctic), Hyriidae (75, South America, Australasia), Etheriidae (4, South America, Africa, SE Asia), Mycetopodidae (43, exclusive from South America), and Iridinidae (43, exclusive from Africa).
INFRACLASS PTERIOMORPHIA
(30:294/)2,313 spp. worldwide (Catalogue of Life), fully marine, (19:77/)130 in Brazil (Machado, FM et al., Zoologia, 2023). Two families includes freshwater members: Myrtillidae (5 spp. in E Asia) and Arcidae (5 spp. in SE Asia).
SUBCLASS PROTOBRANCHIA
(14:82/)878 spp. worldwide (Catalogue of Life), fully marine, (11:27/)54 in Brazil (Machado, FM et al., Zoologia, 2023).
Condylonucula maya D.R. Moore, 1977 (Protobranchia, Nuculidae) is believed to be the smallest living bivalve (500 μm), found in shallow waters in the Caribbean Sea off the coast of Mexico.
GASTROPODA
(568:7,848/)84,015 living spp. worldwide and (265:932/)2,737 in Brazil, numbers based on a compilation of the data below analyzed by clade. Approximately 46,000 marine, 6,000 in freshwater and 32,000 in lands (Molluscabase). For freshwater Gastropods, Strong et al. (Hydrobiology, 2008) cites 3,795 to 3,972 spp. worldwide, mainly in Asia, but 440 to 533 in Neotropics, in 14 to 17 families.
Salvador, RB et al. (Journal of Conchology, 2024) cites (35:126/)715 spp. of native land snails in Brazil (and 24 genera composed only by exotic species). Machado, FM et al. (Zoologia, 2023) cites 1,837 marine Gastropoda and (10:34/)177 spp. in freshwater in Brazil.
Angustopila psammion Páll-Gergely, Vermeulen & Anker, 2022 (Hypselostomatidae) from N Laos is tiniest land snail ever known, with a shell width of 0.6–0.68 mm and a shell height of 0.46–0.57 mm (Páll-Gergely, B. et al., Contributions to Zoology, 2022). Megalobulimus popelairianus Nyst, 1845 (Strophocheilidae), from Brazil and Ecuador, has the largest eggs of all land gastropods: 51 ✕ 35 mm (Wikipedia). Possibly 317 off 327 spp. of Amastridae, a Hawaiian family of terrestrial snails, are extincts today (Régnier, Conservation Biology, 2015). All bioluminous Mollusca presently known are marine organisms, except the gastropods Latia neritoides J.E. Gray, 1850 (Latiidae), a freshwater from northern island of New Zealand, and Quantula striata Gray, 1834 (Dyakiidae), a terrestial snail from Singapore, Malaysia, Cambodia, Philippines, Fiji, and some islands in the Rhio Archipelago (Shimomura & Yampolsky, BOOK, 2019).
Gastropoda's most recent classfication includes the following topology, based on Cunha TJ, Giribet G. (Proc. R. Soc., 2019), with five well defined groups.
A1 PSILOGASTROPODA/PATELOGASTROPODA
Marine, true limpets, with flattened, cone-shaped shells, and the majority of species are commonly found adhering strongly to rocks or other hard substrates. (10:47/)421 spp. worldwide (Catalogue of Life), (3:3/)6 in Brazil (Machado, FM et al., Zoologia, 2023).
A2 PSILOGASTROPODA/VETIGASTROPODA
Marine, primitive snails, widely distributed in all oceans of the world (includes Neomphalida and Cocculiniformia). (47:535/)5,191 spp. worldwide (Catalogue of Life), (20:76/)190 in Brazil (Machado, FM et al., Zoologia, 2023).
B NERITOMORPHA
Includes sea snails, limpets, freshwater snails, land snails and slugs. (8:104/)1,375 spp. worldwide (Catalogue of Life), (4:9/)48 in Brazil (Machado, FM et al., Zoologia, 2023).
Two terrestrial families in Brazil: Proserpinidae (1/1) and Helicinidae (2/34), in Salvador, RB et al. (Journal of Conchology, 2024).
C1 APOGASTROPODA/CAENOGASTROPODA
Mostly sea snails and other marine gastropod mollusks, but also includes some freshwater snails and some land snails. (194:3,426/)39,165 spp. worldwide (Catalogue of Life), (135:491/)1,289 in Brazil (Machado, FM et al., Zoologia, 2023).
Three families inhabits land environments in Brazil: Diplommantinidae (2/6), Megalomastomatidae (2/9) and Neocyclotidae (3/7), in Salvador, RB et al. (Journal of Conchology, 2024). (5:12/)115 freshwater species occur in Brazil (Machado, FM et al., Zoologia, 2023)
C2 APOGASTROPODA/HETEROBRANCHIA
Includes Opisthobranchia (all marine species) and Pulmonata (majority of land snails and slugs, many freshwater snails, and a small number of marine species). (309:3,736/)37,863 spp. worldwide (Catalogue of Life), (103:353/)1,130 in Brazil (Machado, FM et al., Zoologia, 2023).
Includes (30:116/)658 terrestrial species in Brazil, in Salvador, RB et al. (Journal of Conchology, 2024), and (5:21/)62 freshwater species in country (Machado, FM et al., Zoologia, 2023), all in Hygrophila clade of Pulmonata..
By Ramos et al. (Frontiers in Sustainable Food Systems, 2021), terrestrial slugs are not a monophyletic group, but a case of convergent evolution in which the slug form evolved from different lineages of land snails that gradually lost their shell, through a process called limacization. The slug body form is present in the Stylommatophora (land snails and slugs) and Systellommatophora (aquatic and terrestrial slugs) clades of the Eupulmonata; only two families are native to the New World: Veronicellidae (common in tropical areas around the world) and Philomycidae (native from Asia and North America). Thomé (Biociências, 1993) reviewed the native Veronicellidae in the Americas, mentioning 144 species names classified in 18 genera, 10 in Brazil (none endemic), 8 not recorded in, following Oliveira (Dissertation, 2019): Colosius (5, Colombia to Peru and Hispaniola), Diplosolenodes (6, USA, Caribbean, Nicaragua, Guyana, Venezuela and Ecuador), Forcartulus (1, Venezuela and Colombia), Heterovaginia (1, Peru), Leydiula (10, USA to Colombia), Microveronicella (1, Colombia and Ecuador), Montivaginullus (1, Peru) and Veronicella (6, Caribbean, Ecuador and Chile). Despite not having endemic genera, Brazil has the greatest diversity of genera in the New World in this family. In Salvador, R.B. et al. (Journal of Conchology, 2024), Veronicella occurs natively in Brazil.
The family Rathouisiidae, sister to Veronicellidae, comprises slugs native to SE Asia and Australia (Wikipedia). However, the iNaturalist platform lists three possibly native records in the New World: two in SE USA (SEE | SEE) and one in caves near Montes Claros municipality, SE Brazil (SEE).
For sea slugs from Rio Grande do Norte state (in Acteonoidea, Nudipleura, Euopisthobranchia/Anaspidea and Panpulmonata/Sacoglossa, all Heterobranchia), see Delgado, M. et al. (Pap. Avulsos Zool., 2022). For notes from Brazilian Nudibranchia, see Padula (SBZ, 2014).
SCAPHOPODA
(17:52/)617 spp. worldwide (Catalogue of Life), (6:19/)42 spp. in Brazil (CTFB/Scaphopoda), in both two orders of this class: Dentaliida (3:9/19) and Gadilida (3:11/23).
▉ endemic families in New World: Globocornidae (1/1, Gastropoda/Caenogastropoda, Cuba) and Amastridae (8/20, Gastropoda/Heterobranchia, Hawaii, USA).
▉ LAST UPDATE OF MOLLUSCA IN JANUARY/FEBRUARY 2026
18. ANNELIDA ‣ the traditional classification of Annelida into three main groups is now obsolete. The most recent and stable phylogenetic framework was proposed by Capa & Hutchings (Diversity, 2021), who identified 18 distinct lineages within Annelida. This includes taxa previously treated as separate phyla (Echiura and Sipuncula). After their study, three additional lineages were proposed: Protodriliformia (SEE), and Aeolosomatida/Hrabeiellidae (Schmelz et al., Zootaxa, 2021) totaling 20 lineages, the highest number of canonic lineages in a single phyllum. CTFB recognizes the groups Echiura (SEE) and Sipuncula (SEE) as separate from Annelida.
Grosse et al. (Diversity, 2021) mention several groups with unresolved phylogenetic placement within Annelida, namely: Cossuridae, Paraonidae, Siboglinidae, Hrabeiella, Aeolosoma, Potamodrilus, Scalibregmatidae, and Travisiidae. The last six are tentatively placed within the lineages proposed by Capa & Hutchings (2021), while Cossuridae and Paraonidae remain 'unplaced at lineages' due to insufficient phylogenetic evidence. Another group with unresolved placement is Myzostomida, as noted by Summers & Rouse (BMC Evolutionary Biology, 2014), for which the consulted literature provides no conclusive positioning. Grosse et al. (2021) also refer to 'many other' unresolved groups within Annelida without providing a specific list.
According to our data, Annelida includes (163:2,465/)23,051 spp. worldwide and (98:580/)1,877 occurring in Brazil. Mexico includes (63:460/)1,500 spp. of former Polychaetes (Tovar-Hernández, Revista Mexicana de Biodiversidad, 2014).
Of the global total, 13,738 species inhabit marine environments (Frontier Sin/2023). A literature review of former Polychaeta (including oligochaete-like Aeolosomatidae and Potamodrilidae), living in freshwater yielded (24:70/)168 spp. representing all of the major polychaete clades, but less than 2% of all species (Gçasby & Tarmo, Hydrobiologia, 2008). Terrestrial Annelida worldwide belongs to Clitellata except Hrabeiella (from Europe and Korea, Farkas, Environmental Science Biology, 2013).
The number of former polychaete taxa inhabiting Neotropical freshwater environments is very small compared to their marine counterparts, comprising only around (6/)22 spp. (KNAF, 2020) in five families: Nereididae (2/11, in Nemalycastis and Nemanereis, both genera recorded in Brazil, which has about 10 spp., mainly from Amapá to Maranhão | Alves, Thesis, 2022), Histriobdellidae (8, all in Stratiodrilus, collected in various locations including Brazil), Capitellidae (1/1, from the Ouanary River in French Guiana), Sabellidae (1, Monroika clarae Bick & Armendáriz, 2021, from the lower Uruguay River in Argentina and Uruguay, SEE), and Sigalionidae (1, Phyllodocida, from Panama).
Some checklists of Annelida worldwide includes Polychaeta from the continental shelf off Aveiro in NW Portugal (37:136 spp., Ravara & Moreira, Check List, 2013), Polychaeta from Malaysia (32:64 spp., Idris & Arshad, Asian Journal of Animal and Veterinary Advances, 2013), Polychaeta from Gulf of Paria from Trinidad & Tobago (38:188 spp., Gobin, Caribbean Marine Studies, 1990), Polychaeta from Caribbean coast of Colombia (51:230/293 spp., Victoria Leon, M et al., Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales, 2019), Clitellata/Oligochaeta from Hispaniola (22 spp., Álvarez-Abreu & Carrera-Martínez, Zootaxa, 2025), Clitellata/Oligochaeta from Algeria (8:20/40 spp., Chergui, CN et al., Zootaxa, 2025).

PHYLOGENY OF ANNELIDA BY CAPA & HUTCHINGS (DIVERSITY, 2021), PLUS PROTODRILIFORMEA AND AEOLOSOMAITA/HRABEIELLIDAE
All recent and valid families recognized by the Catalogue of Life/Annelida are represented here with their respective numbers, except Sipuncula (treated as a separate phylum in this sources), Hrabeiellidae, and Eenymeenymyzostomatidae. For Brazil, all numbers presented here follow the CTFB/Annelida, except for Myzostomidae, Siboglinida, Aberrantidae, Echiura (treated as an independent phylum), Scalibregmatidae, and exclusion of families in Crassiclitellata and Hirudina.
INCERTAE SEDIS
11 families are currently unplaced in established annelid lineages with (30/)275 spp. worldwide. Three of these are represented in Brazil (10/51): Cossuridae (1/30 | 1/5 in Brazil), Paraonidae (8/187 | 6/42 in Brazil) and Myzostomidae (12/172 | 3/4 in Brazil, not listed in CTFB, but accepted here Summers & Rouse, BMC Evolutionary Biology, 2014). 8 families do not occur in Brazil: Asteriomyzostomidae (1/2, PI, Mediterranean and Pacific), Asteromyzostomidae (1/1), Endomyzostomatidae (3/5), Eenymeenymyzostomatidae (1/2), Protomyzostomidae (1/5), Pulvinomyzostomidae (1/3), Laetmonectidae (1/1 | WORMS) and Spintheridae (1/12 | Wikipedia).
PALAEOANNELIDA
(6/)138 spp. worldwide in two families (Parapar, Diversity, 2021), both in Brazil (5/18): Magelonidae (2/83 | 1/8 in Brazil) and Oweniidae (4/55 | 4/10 in Brazil).
CHAETOPTERIDAE
(5/)84 spp. worldwide in a single homonymous family. (4/)8 spp. in Brazil.
AMPHINOMIDA
(28/)223 spp. in two families worldwide, both in Brazil (11/20): Amphinomidae (24/195 | 10/18 in Brazil) and Euphrosinidae (4/64 | 1/2 in Brazil).
SIPUNCULA
(6:33/)153 spp. worldwide (Catalogue of Life). All families occur in Brazil (12/39, CTFB/Sipuncula). For some notes for Sipuncula in NE Brazil, see Franco, LC et al. (Zootaxa, 2024).
EUNICIDA
(9:119/)1,515 spp. worldwide — but we highlight Amphinomidae under Amphinomida and include Histriobdellidae. Six families occur in Brazil: Dorvilleidae (38/241 CL | 7/15), Eunicidae (12/501 | 7/74), Histriobdellidae (4/14 | 1/5), Lumbrineridae (21/293 | 11/29), Oenonidae (18/100 | 7/14), and Onuphidae (23/355 | 11/56). In total, (6:44/)203 spp. are known from Brazil.
Exallopus hilbigae (Dorvilleidae) is a new species from Brazil (Bonaldo, RO et al, Systematics and Biodiversity, 2025), not been counted in the numerical totals of this study.
Three families are unknown in Brazil: Diurodrilidae (1/7), Hartmaniellidae (1/3) and Ichthyotomidae (1/1).
PHYLLODOCIDA
(26:590/)5,076 spp. worldwide, (22:170/)552 are known from Brazil. Martin (2021) cites 28 families, but here we mainly follow Wikipedia, which lists 26 families (SEE), with the tentative addition of Microphthalmidae and Iphionidae, and exclusion of Alciopidae, Lepidocoleidae, Nautiliniellidae, Pholoidae, Pisionidae, Plumulitidae and Turrilepadidae after Catalogue of Life, resulting in a total of 26 valid families. 22 of them occur in Brazil: Acoetidae (12/61 | 5/10), Aphroditidae (8/119 | 4/6 in Brazil), Chrysopetalidae (31/114 | 4/7 in Brazil), Eulepethidae (11/25 | 3/8 in Brazil), Glyceridae (6/85 | 3/17 in Brazil), Goniadidae (9/88 | 8/22 in Brazil), Hesionidae (40/241 | 10/19 in Brazil), Iospilidae (3/5 | 2/3 in Brazil), Lacydoniidae (1/16 | 1/7 in Brazil), Lopadorrhynchidae (8/22 | 4/6 in Brazil), Microphthalmidae (8/61 | 1/1 in Brazil), Nephtyidae (5/154 | 3/19 in Brazil), Nereididae (52/807 | 19/79 in Brazil), Paralacydoniidae (1/2 | 1/1 in Brazil), Phyllodocidae (36/516 | 17/64 in Brazil), Pilargidae (12/125 | 8/24 in Brazil), Polynoidae (192/936 | 18/42 in Brazil), Sigalionidae (34/262 | 16/42 in Brazil), Sphaerodoridae (12/134 | 1/1 in Brazil, Syllidae (102/1,231 | 38/172 in Brazil), Tomopteridae (4/55 | 1/8 in Brazil), and Typhloscolecidae (4/17 | 3/4 in Brazil).
Four families does not occur in country: Antonbruuniidae (1/3, Iphionidae (4/24), Pontodoridae (1/1) and Yndolaciidae (3/3). In total,
Among all Annelida, only three branching species with a highly modified body-pattern are known until now, all in Syllidae within Phyllodocida: Syllis ramosa McIntosh, 1879 (250 m near the Philippines and at a depth of 170 m in the Arafura Sea), Ramisyllis multicaudata Glasby et al. (inside both white and purple sponges of the genus Petrosia in Darwin Harbour, Australia), and R. kingghidorahi Aguado, Ponz-Segrelles, Glasby, Ribeiro, Jimi & Miura, 2022, from Shukunegi Point, at the southern tip of Sado Island, Japan. All have unusual ramified bodies with one head and multiple anuses (Wikipedia), and live inside the canals of host sponges (Species New to Science).
The highest record of a freshwater non-Clitellata Annelida in world belongs Lycastoides alticola Johnson, 1903, found at 2,150 m asl in Mexico (Conde-Vela, Subterranean Biology, 2017).
PROTODRILIFORMIA
(15/)107 spp. worldwide in five families, four in Brazil: in Protodrilidae (7/40 | 2/2 in Brazil), Polygordiidae (2/21 | 1/3 in Brazil), Saccocirridae (2/24 | 2/3 in Brazil) and Protodriloididae (1/2 | 1/1 in Brazil). In total, (4:6/)9 spp. are known from Brazil. Dinophilidae (3/20 | Wikipedia) does not occur in Brazil.
ORBINIIDA (includes Questidae)
(52/)345 spp. worldwide in four families (Wikipedia). Two occur in Brazil (10/36): Orbiniidae (31/274 | 9/35 in Brazil) and Nerillidae (18/57 | 1/1 in Brazil). Apharyngtidae (1/1, Worsaae, K et al., Diversity 2021) and Parergodrilidae (2/13) does not occur in Brazil.
Parergodrilidae includes 13 spp. (Purschke & Fursman, Zoomorphology, 2005): Parergodrilus heideri Reisinger, 1925 (terrestrial, living in the zone of leaf litter and has so far only been found in Europe) and 12 Stygocapitella, in North America, E Asia, Europe, South Africa, Australia, and New Zealand (Meca, MA et al., Diversity 2021).
CIRRATULIFORMIA
(8:134/)1,171 spp. worldwide and (7:33/)74 in Brazil. Although Terebellidae and Trichobranchidae are included in this group on Wikipedia, we assign them to Terebelliformea (Hutchings et al., Diversity, 2021) in this work. Consequently, this group is accepted here with 8 valid families, Alvinellidae (2/12) not recorded in Brazil, and 7 in country: Acrocirridae (10/45 | 1/3 in Brazil), Ampharetidae (57/288 CL | 4/6 in Brazil, Cirratulidae (22/417 | 10/36 in Brazil), Fauveliopsidae (4/26 | 3/3 in Brazil), Flabelligeridae (27/261 CL | 8/16 in Brazil), Pectinariidae (5/74 | 4/7 in Brazil), and Sternaspidae (7/48 | 3/3 in Brazil). The families Ctenodrilidae, Flotidae, and Poeobiidae are commonly cited as valid, but here, following the Catalogue of Life, they are considered invalid.
The Alvinellidae are a family of worms that are endemic to deep-sea hydrothermal vents in the Pacific and Indian Oceans (Molecular Phylogenetics and Evolution, 2024). Alvinella pompejana Desbruyères and Laubier, 1980 (Alvinellidae) is the most heat-tolerant complex organism known on Earth, found near hydrothermal vents deep in Galapagos vents, it thrives at a temperature of 50 °C; this is near the theoretical limit for eukaryotes, whose mitochondria disintegrate at about 55 °C (Wikipedia).
SIBOGLINIDAE
(34/)214 spp. worldwide in a single family homonymous and 4 lineages, Frenulata clade (FC), Osedax clade (OC), Vestimentifera, and Monilifera (Sclerolinum, SEE). Brazil includes five spp.: Osedax braziliensis Fujiwara, Jimi, Sumida, Kawato & Kitazato, 2019 (OC, SEE), O. nataliae Gularte, Sumida, Bergamo & Rouse (OC, Gularte, T. et al., ZooKeys, 2024), Siboglinum besnardi Tommasi, 1970 (FC, SEE), S. nonatoi Tommasi, 1970 (FC, SEE) and Crassibrachia brasiliensis Southward, 1968 (FC, SEE). Vestimentifera and Monilifera do not occur in Brazil.
Vestimentifera has six genera endemic to hydrothermal vents in the Pacific(Riftia, Ridgeia, Tevnia, Oasisia, Alaysia, and Arcovestia) and four widely distributed (Lamellibrachia, Escarpia, Paraescarpia and Seepiophila), based in McCowin et al. (Molecular Phylogenetics and Evolution, 2023).
SABELLIDAE
(144/)1,408 spp. worldwide in four families, three in Brazil (47/88): Fabriciidae (17/90 | 3/3 in Brazil), Sabeliidae (46/595 | 23/42 in Brazil) and Serpuliidae (80/721 | 21/43 in Brazil). Potamodrilidae (1/2) does not occur in Brazil.
SABELARIIDA
(14/)156 spp. worldwide in a single family Sabelariidae, with (6/)19 spp. in Brazil.
SPIONIDA
(61/)813 spp. in eight families worldwide (Magelonidae and Chaetopteridae have been moved to other lineages). (6:27/)114 spp. in Brazil, in Aberrantidae (1/5 | 1/1 in Brazil, Santos & Rizzo, Ocean and Coastal Research, 2024), Apistobranchidae (1/7 | 1/1 in Brazil), Longosomaridae (1/24 | 1/1 in Brazil), Poecilochaetidae (1/34 | 1/7 in Brazil), Spionidae (51/714 | 21/102 in Brazil), and Trochochaetidae (2/13 | 2/2 in Brazil). Uncispionidae (3/8) and Psammodrilidae (1/8 | Wikipedia) are unknown in Brazil.
CAPITELLIDA/ECHIURA
(7:134/)711 spp. worldwide, and (4:38/)81 in Brazil. Due to high inconclusion among phylogenies, this clade is treated here including two groups: Echuira and Capitellida.
A. Echiura ‣ (48/)186 spp. worldwide in five families of two orders (Wikipedia): Bonelliidae (30/78), Echiuridae (1/4), Ikedidae (1/2), Thalassematidae (7/85) and Urechidae (1/4). (2:8/)9 spp. in Brazil, 7 listed in CTFB plus Torbenwolffia galatheae Zenkevitch, 1966 (Bonellidae) and Sluiterina flabellorhynchum Murina, 1976 (Bonellidae), listed in Biseswar (Zootaxa, 2009). Other three South American species are listed in Paz & Chapoñán (Cientifica, 2016).
B. Capitellida ‣ (86/)525 spp. in two families worldwide, both in Brazil (2:30/72): Capitellidae (45/235 | 11/36 in Brazil) and Maldanidae (41/290 | 19/36 in Brazil). Originally included three families (Wikipedia), but with the emancipation of Arenicolidae, only two are in this group today.
SCALIBREGATIDAE/TRAVISIDAE
(18/)193 spp. worldwide in two families: Scalibregmatidae (17/147) and Travisiidae (1/46). (5/)17 spp. in this clades in Brazil: 12 in Scalibregmatidae (EJT, three in Asclerocheilus, three in Oligobregma, three in Pseudoscalibregma and also three in Scalibregma), and (1/)5 in Travisidae (CTFB).
OPHELIIDA
(10/)180 spp. worldwide in a single family, Opheliidae. (7/)32 spp. in Brazil.
ARENICOLIA
(6/)25 spp. in a single family, Arenicolidae. (2/)4 spp. occur in Brazil.
TEREBELLIFORMEA
(76/)845 spp. in three families worldwide (Diversity, 2021 | Guton, L. et al., Records of the Australian Museum, 2023), all in Brazil (28/80): Melinnidae (4/57 | 2/3), Terebellidae (68/667 | 23/67), and Trichobranchidae (4/121 | 3/10). Polycirridae, Thelepodidae and Telothelepodidae commonly cited as valid, but here, following the Catalogue of Life, they are considered invalid.
CLITELLATA
A spectacular assemblage of distributed across 20 orders (Martin, P et al, Zoological Journal of the Linnean Society, 2024 | Tessler, Molecular Phylogenetics and Evolution, 2018) and more than 70 families, which can be grouped into 10 major lineages, two of them monotypic and endemic to Japan. For a huge amount of informations about earthworms, see Zootaxa/VOL 5255, 2023.
According to our data, there are (59:952/)9,370 spp. worldwide and (20:111/)416 occurring in Brazil, in 9 orders. 11 orders remains unknown in Brazil.
Only 13 of the approximately 600 known species of marine and estuarine Clitellata have been recorded from Brazil, and 8 of them are currently known only from their type localities (Prantoni et al., Marine Biodiversity, 2013), in Naididae (5/6), Enchytraeidae (3/5), Capilloventridae (1, Capilloventer atlanticus Harman & Loden, 1984), and Megascolecidae (Pontodrilus litoralis Grube, 1855). In addition to being the aquatic oligochaete with the highest number of records in Brazil, P. litoralis is considered invasive here (Misirlioglu et al., Zootaxa, 2023).
Bioluminescent Clitellata are found among approximately 30 spp. across five families (Shimomura & Yampolsky, Bioluminescence, 2019): Acanthodrilidae, Megascolecidae, Octochaetidae, Lumbricidae (all known cases of bioluminescence are in Eisenia, and the phenomenon may be restricted to just two or three species, including E. fetida Savigny, 1826 and E. lucens Waga, 1857), and Enchytraeidae (in Fridericia and Michaelseniella, which typically inhabit soils rich in organic matter).
UNPLACED ORDERS
ORDER NARAPIDA
A single freshwater species in Narapidae, Narapa bonettoi Righi & Varela, 1983, from S Brazil to NE Argentina (Jamieson et al., Book Chapter 8, 2006).
ORDER MONILIGASTRIDA
A single family, Moniligastridae, with (5/)189 spp. worldwide (Catalogue of Life), from SE and E Asia. They are terrestrial and, despite a common belief that they are amphibious, no moniligastrids are known to be aquatic or limicolous.
PARVI/RANDIELLI CLADE
ORDER RANDIELLIDA
One small marine family, Randiellidae, including 4 spp. in a single genus, Randiella (Timm, Zootaxa, 2017): one species is known from the NE Pacific Ocean (Oregon), the three from the NW Atlantic (east coast of USA and Caribbean) and New Caledonia (Erséus, Journal of Natural History, 1997).
ORDER PARVIDRILLIDA
A single family, Parvidrilidae (1/11, freshwater), from SE USA and Europe (Timm, Zootaxa, 2017).
CAPILOVENTRIDA CLADE
ORDER CAPILLOVENTRIDA
A single family, Capilloventridae (6) in a single genus Capilloventer: two marine (C. atlanticus Harman & Loden 1984 from E Brazil and one in Antarctica) and 4 freshwater species in Australia (KAFTI).
EH CLADE
ORDER ENCHYTRAEIDA
Two families and (45/)954 spp. worldwide, in Enchytraeidae with (44/)951 spp. worldwide (mostly terrestrial, also freshwater, marine), 65 in South America and (9/)36 in Brazil; and Propappidae (1/3, freshwater), from Europe and S Siberia in Russia (Coates, Proceedings of ohe Biological Society of Washington, 1986).
ORDER HAPLOTAXOIDIDAE
(1/)5 spp. in a single family, Haplotaxoididae, endemic to USA, freshwater.
TUBIFICIDA CLADE
ORDER TUBIFICIDA
Two families and (161/)1,344 spp. worldwide in Naididae (151/1,285 | including Opistocystidae, 26/80 in Brazil) and Phreodrilidae (10/59 unknown in Brazil).
HAPLOTAXIDA CLADE
ORDER HAPLOTAXIDA
14 spp. in two monotypic families: Haplotaxidae (freshwater 2/2 in Brazil), and Tiguassuidae (1/1, endemic to N Brazil, freshwater). (2:3/)3 spp. in Brazil.
CAP CLADE
ORDER ALLUROIDINA
Two families, Alluroididae (7/12, amphibious, mainly freshwater, Jamieson & Fragoso, Zootaxa, 2024, waters of equatorial Africa and South America) and Syngenodrilidae (1/1, terrestrial, E Africa). The former family has (4/)5 spp. in New World, one in Mexico (Lacandodrilus), (3/)3 in Guyana (Kathrynella and Righiella endemic genera), and Brinkhurstia americanus Brinkhurst, 1964 in Brazil, Guyana, Argentina and Santa Lucia in Caribbean.
ORDER PELODRILIDA
(7/)16 spp. in a single family, Pelodrilidae, range unknown.
ORDER CRASSICLITELLATA
(22:468/)5,862 spp. worldwide. Here we consider all Crassiclitellata families recognized by the Catalogue of Life, except Octochaetidae, which is treated here as part of Acanthodrilidae, following Misirlioglu et al. (Zootaxa, 2023), and Hippoperidae, synonimized in Eudrilidae (Glasby, CJ et al., ZooKeys, 2025).
Nine families do not occur in New World: Criodrilidae (Europe and Algeria), Biwadrilidae (Japan), Diporodrilidae (Corsica and Sardinia), Eudrilidae (Africa), Hormogastridae (Europe and Northern Africa), Kazimierzidae (Western Cape and Northern Cape provinces), Kynotidae (22, Madagascar), Microchaetidae (South Africa), and Tritogeniidae (southern Africa). 4 families occur in New World but non in South America: Komarekionidae (mid-Atlantic states west to southern Illinois, USA), Lutodrilidae (E USA), Megascolecidae (Asia, Australia, New Zealand, Canada to California), and Sparganophilidae (North America).
Nine families occur in South America. CTFB lists Criodrilidae, Megascolecidae, and Eudrilidae as occurring in Brazil, but all of them are rejected here. Arecoidae is not listed in CTFB, but is accepted in this work. (7:57/)249 spp. in Brazil.
ACANTHODRILIDAE ‣ (84/)956 spp. worldwide (including Octochaetidae), widely worldwide, 66 in South America, only (2/)4 in Brazil.
ARECOIDAE ‣ a single spp., Areco reco Righi, Ayres and Bittencourt, 1978, endemic to N Brazil, dismembered of Almidae.
ALMIDAE ‣ (7/)68 worldwide, in Africa, SE Asia, Costa Rica to South America and the Caribbean, (3/)9 spp. in New World, (3/)3 in Brazil.
BENHAMIIDAE ‣ (16/)389 spp. worldwide, in Africa, Oceania, America Central and northern South America. (5/)100 spp. in New World, including former South American Octacaenidae. (4/)20 spp. in Brazil.
GLOSSOSCOLECIDAE ‣ (17/)200 spp. worldwide, from Mexico to South America, (7/)62 in Brazil (Diaguita-2, Enantiodrilus-1, Fimoscolex-7, Glossodrilus-4, Glossoscolex-27, Holoscolex-2, and Righiodrilus-19) remaining genera in CTFB/Annelida are placed in Rhinodrilidae.
LUMBRICIDAE ‣ (63/)777 spp. worldwide. A family with the native status in Brazil quite unclear. Christoffersen (Zoosystema, 2011) lists 26 spp. in South America, with only two being restricted to the continent. (7/)12 spp. in Brazil in CTFB/Annelida, number accepted here.
OCNERODRILIDAE ‣ (40/)179 spp. worldwide, South and America Central, sub-Saharan Africa, India, and the Seychelles. (20/)77 spp. in South America, (15/)45 spp. in Brazil.
RHINODRILIDAE ‣ (46/)439 spp. worldwide (under Glossoscolecidae in CTFB/Annelida) from Mexico to South America.(18/)102 in Brazil (excluding the 7 genera of Glossoscolecidae).
TUMAKIDAE ‣ (1/)3 spp., endemic to Colombia.
OHTAKIANIDA CLADE
ORDER OHTAKIANIDA
A single species also endemic to Japan, Ohtakianidae, freshwater.
LIMPLUVIDA CLADE
ORDER LIMPLUVIDA
A single species endemic to Japan, Limpluvidae, freshwater.
LUBRICULIDA CLADE
ORDER LUMBRICULIDA
(2:36/)266 spp. in two families, Lumbriculidae (35/263) and Dorydrilidae (1/3, freshwater), mostly freshwater and Holarctic with extension into W Asia, not recorded in South America.
HIRUDINEA
ORDER BRANCHIBDELLIDA
(23/)157 spp. in a single family, Branchiobdellidae, obligate ectosymbionts primarily associated with astacoidean crayfishes, in freshwater habitats from Canada to SE Mexico in Veracruz state, also isolated records in Nicaragua and Costa Rica (Gelder, Hydrobiologia, 1999), Euro-Mediterranean, and E Asia (Gelder & Williams, Thorp and Covich's Freshwater Invertebrates, 2015). (6/)17 spp. in Mexico (Almanaque Z).
ORDER ACANTHOBDELLIFORMES
Three known species in a single family, Acanthobdellidae, in Acanthobdella (2, broadly across northern Eurasia and Alaska) and Paracanthobdella livanowi Epstein, 1966 (Russian Far East), in De Carle et al. (Zoological Journal of the Linnean Society, 2022).
ORDER RHYNCHOBDELLIDA
(3:114/)324 spp. worldwide and (8/)32 in Brazil, in all families: Glossiphoniidae (3/26 in Brazil), Ozobranchidae (1/1 in Brazil) and Piscicolidae (4/5).
ORDER EUHIRUDINEA
A single family, Salfidae (7/12 worldwide), from Eurasia, Africa, Madagascar, Australasia, and Australia (Bolotov et al, Ecologica Montenegrina, 2023), unknown in New World.
ORDER ARHYNCHOBDELLIDA
(12:66/)185 spp. worldwide and (3:5/)13 spp. in Brazil (CTFB/Arhynchobdellida), in Cylicobdellidae (1/4 in Brazil), Hirudinidae (2/4 in Brazil, including Macrobdellidae, sometimes assignated as absent in New World — Paleartic in these sources, SEE), and Semiscolecidae (2/5 in Brazil).
9 families has no native records from Brazil, being Gastrostomobdellidae and Haemadipsidae from E Asia (SEE), Americobdellidae (endemic to Chile, SEE), Xerobdellidae (Mesobdella in Chile, Xerobdella in Europe, and Diestecostoma in Central and northern South America, Wikipedia), Erpobdellidae (N Canada to S Mexico and Eurasia — Anderson, K et al, Molecular Phylogeny and Evolution, 2020), Haemopidae (swaths of continental Europe and the British Isles to central Asia, and from Canada and USA to C Mexico — Kvist, S et al., Molecular Phylogenetics and Evolution, 2023), Orobdellidae (1/21, Japan, Taiwan, Korea and E Russia, Wikipedia), and Praobdellidae (Africa to Asia, Mexico, America Central and Peru — Phillips et al, Plos One, 2010 | Schenková, J et al, Parasitology International, 2021). (9:)30 spp. in Mexico (Oceguera-Figueroa & León-Règagnon, Rev. Mex. de Biodiv., vol. 85, 2014, excludes Salifidae). Mexican families never collected in Brazil: Erpobdellidae (3, SEE), Haemopidae, Praobdellidae, and Xerobdellidae. Cylicobdellidae unknown in Mexico.
AEOLOSOMATIDA/HRABEIELLIDAE
Two families (4/49), Aeolosomatidae with (3/)47 spp. worldwide and Hrabeiellidae (1/2, Hrabeiella, only known exclusively terrestrial non Clitellata by Farkas, Environmental Science Biology, 2013, been found at an increasing number of sites in Europe and has also recently been reported from Korea), only the former in Brazil (1/11).
▉ endemic families in New World: Americobdellidae (1/1, Clitellata, Chile), Tumakidae (1/3, Clitellata, Colombia), Komarekionidae (1/1, Clitellata, USA), Lutodrilidae (1/1, Clitellata, USA), Haplotaxoididae (1/5, Clitellata, USA), Arecoidae (1/1, Clitellata, Brazil) and Tiguassuidae (1/1, Clitellata, Brazil).
▉ LAST UPDATE OF ANNELIDA IN JANUARY/FEBRUARY 2026
19. BRACHIOPODA ‣ (31/121)409 living species into five orders (Brachiopoda Database) and (8:9/)11 spp. in Brazil, all marine, explaned below in three groups.
LINGULIFORMEA
(2:6/)25 spp. worldwide, five genera in New World: Glottidia (MAP), Pelagodiscus (MAP), Lingula (SEE), Discinisca (MAP) and Discradisca (MAP), all in South America, the three lasts in Brazil in both families: a unnamed Discinisca (Simões et al., Paleontology, 2004), Dicradisca antillarum d'Orbigny, 1845 (Collin et al., Diversity, 2019) and an unnamed Lingula (Monteiro et al., Labomar, 2023).
The sixth genus, Discina, occur only in W Africa (MAP).
RHYNCHONELLIFORMEA
Three orders and (28:112/)371 spp. worldwide.
RHYNCHONELLIDA
19 genera, three in New World: Cryptopora (7, North Atlantic from Scandinavia to Morocco, Panama and Caribbean, South Georgia, S Australia and New Zealand, MAP), Abyssorhynchia (1, Hawaii and Pacific coast from Panama to Chile, MAP) and Neorhynchia (1, Indonesia to New Zealand, Alaska to Chile and aroud Antarctica, MAP), all in South America, unknown in Brazil.
THECIDEIDA
Six genera, three in New World, all only in Caribbean Basin (remaining records in Old World, unknown in Colombia), but also possibly in N South America: Lacazella (3, Mediterranean, SE Africa and Caribbean, MAP), Minutella (4, Red Sea, SE Africa, Japan to Borneo and New Zealand, and Caribbean, MAP) and Thecidellina (12, Red Sea, SE Africa, Japan to Java and New Zealand, French Polynesia and Caribbean, MAP), unknown in Brazil.
TEREBRATULLIDA
87 genera, 15 in South America: Abyssothyris (MAP), Aneboconcha (MAP), Argyrotheca (1 in Brazil, SEE), Bouchardia (SEE), Chlidonophora (Wikipedia, in Guyana), Dallithyris (MAP), Fosteria (MAP), Gryphus (MAP), Liothyrella (MAP), Macandrevia (MAP), Magellania (MAP), Melvicallatis (MAP), Platidia (MAP), Terebratella (MAP), Terebratullina (MAP), and Tichosina (MAP).
Besides these references, in Brazil occur (5:5/)7 spp. at this orders (Simões et al., Paleontology, 2004 | César Marques, Marine Biodiversity, 2018 | César Marques, SEE): Tichosina martinicensis Cooper, 1977 (Terebratulidae), Bouchardia (Bouchardiidae, Simões e Kowalewski, Hystorical Biology, 2009), Platidia anomioides Scacchi & Philippi in Philippi, 1844 (Platidiidae), Argyrotheca cf. cuneata and Argyrotheca sp. (Megathyrididae), Terebratulina cailleti Crosse, 1865 and Terebratulina sp. (Cancellothyrididae).
CRANIIFORMEA
(1:3/)11 spp. worldwide in Craniidae, in Valdiviathyris (1, MAP), Neoancistrocrania (1, MAP) and Novocrania (9, Robinson, Zootaxa, 2017), these collected in New World (3) in SW Canada, W USA, Galapagos, Chile and Magellanic region.
A record of Craniidae off the coast of Brazil is accepted here, based on a 1977 collection by E. Petuch in the Abrolhos region, off the southeastern coast of Bahia, consisting of five individuals (Invertbase/Voos Marine Invertebrate Collection). Unfortunately, the species from this collection was never identified.
■ endemic families in New World: Bouchardiidae (1/1, Rhynchonellata, Brazil).
▉ LAST UPDATE OF BRACHIOPODA IN JANUARY/FEBRUARY 2026
20. BRYOZOA (ECTOPROCTA) ‣ marine and freshwater animals, (236:1,007/)7,037 spp. worldwide in three classes, (105:224/)520 in Brazil, in all classes. A important website for this phylum is International Bryozoology Associaton (IBA). Wood & Okamura (Zootaxa, 2017) lists 89 spp. of freshwater Bryozoa worldwide, 30 in Neotropical regions (24 in Phylactolaemata, and six in Ctenostomata, at Hislopiidae), 16 in Brazil, but the work describes more 4 news from Phylactolaemata (and create two families in Phylactolaemata and Ctenostemata one each). Brazil has, in total, 20 freshwater bryozoa (18 Phylactolaemata and two Ctenostemata).
All data presented below follow Catalogue of Life/Bryozoa at the global level and CTFB/Bryozoa for Brazil, with occasional additions highlighted in the text.
GYMNOLAEMATA
(201:859)6,198 spp. worldwide, including marine and freshwater forms. (91:205/)460 spp. in Brazil.
STENOLAEMATA
(27:130/)723 living spp. worldwide in a single living order, Cyclostomata, all marine. (10:15/)34 spp. in Brazil.
PHYLACTOLAEMATA
(8:18/)110 spp. worldwide, freshwaters. (4:4/)26 spp. in Brazil.
▉ endemic families in New World: Polliciporidae (1/1, Gymnolaemata, Chile), Jebramellidae (1/1, Gymnolaemata, Brazil) and Tapajosellidae (1/1, Phylactolaemata, Brazil).
▉ LAST UPDATE OF BRYOZOA IN JANUARY/FEBRUARY 2026
21. PHORONIDA ‣ (2/)15 spp. worldwide (Temereva and Neklyudov, Biology Bulletin, 2018), five in Brazil: Phoronis ovalis Wright, 1856 (Paleopolis), P. psammophila Cori, 1889 (Paleopolis), P. hippocrepia Wright, 1856 (Paleopolis | these fully assignated in Brazil by Forneris, SBZ, 1987), P. muelleri Selys-Longchamps, 1903 (SEE | dubious in Brazil) and P. australis Haswell, 1883 (SEE | Paleopolis).
Phoronida, along with Placozoa, is one of the two phyla with the most unstable intraphylogenetic situation. Although traditionally recognized in the literature with two genera — Phoronis and Phoronopsis — studies by Temereva and Neklyudov (Biology Bulletin, 2018) showed that Phoronis is highly paraphyletic. This would require either fragmenting the group into several small genera to maintain Phoronopsis as valid, or forming one or two new small genera. Considering that the most basal species occurs in Brazil (P. ovalis), that the group exhibits extreme morphological monotonicity, and that it shows low apparent diversity, it makes more sense in this work to treat Phoronida as a single canonical lineage.
In actual circusncription, Phoronopsis (3) occur in Panama, USA, Bermuda, Caribbean and scattered in Old World , and is unknown in South America.
▉ LAST UPDATE OF PHORONIDA IN JANUARY/FEBRUARY 2026
ECDIZOA
22. LORICIFERA ‣ (2:11/)47 described spp. worldwide (R.C. Neves et al., Zoologischer Anzeiger, 2016 | R.C. Neves et al., Marine Biodiversity, 2018 | R.C. Neves et al., Plos One, 2021 | Sorensen M.V. et al., Zoologischer Anzeiger, 2022 | Sorensen M.V. et al., European Journal of Taxonomy, 2023 | Sørensen et al., Organisms Diversity & Evolution, 2023), collected in N Chile (1, Pliciloricus ukupachaensis Sørensen, Herranz, Grzelak, Shimabukuro, Kristensen & Zeppilli, 2023, Pliciloricidae), SE Brazil off Rio de Janeiro state (1, Scaberiloricus samba Sørensen, Herranz, Neves, Kristensen & Garraffoni, 2023, Pliciloricidae), Galapagos Islands (2, Spinoloricus neuhausi Neves & Kristensen, 2016, S. turbatio Heiner & Neuhaus, 2007, both Nanoloricidae), both coasts of USA (3/9), N Atlantic (1/2), Mediterranean (1/1), Faroe Is. (3/5), Italy (1/1), France (2/3), Spain (1/1), Guinea Gulf (1/1), Namibia (2/3), Comores (1/1), Russia (1/1, Laptev Sea), Japan (2/2), SE Australia (2/2), Papua New Guinea (1/1), Antartic waters (1/1) and New Zealand (1/1). Consistent records also in Mexico (4/4, Cardoso Neves et al., Front. Mar. Sci., 2022).
Only USA, N Atlantic, Galapagos, Atacama trench, SE Mexico, Faroe Islands, France, Namibia, Japan and SE Australia has more a than a single species.
▉ LAST UPDATE OF LORICIFERA IN JANUARY/FEBRUARY 2026
23. KINORHYNCHA ‣ (10:33/)364 spp. worldwide (Catalogue of Life/Kinorhyncha) and (5:6/)8 in Brazil (data below), nominaly in two classes. Gulf of California in Pacific Mexico includes (2:4/)10 spp. of Kinorhyncha (CBM). Mexican part of Gulf of Mexico includes 24 spp. (Zoologischer Anzeiger). In summary, Mexico includes 34 spp. of Kinorrhyncha.
CYCLORHAGIDA
(5:17/)242 spp. worldwide in three subclades: Xenosomata (only Campyloderidae), Kentrorhagata (3 families, including the aberrant Zelinkaderes) and Echinorhagata (1 family). Brazil has at least 4 spp.: Echinoderes ajax Sørensen, 2014, E. marthae Sørensen, 2014 and E. astridae Sørensen, 2014, all collected from coast of São Paulo state (Echinoderidae, Echinorhagata, Sorensen, Fapesp, 2014), and one Centroderis sp. from E coast (GBIF, Kentrohagata).
Xenosomata includes two genera in Campyloderidae: Ryuguderes (2, Okinawa, Japan, and west of Madagascar, Sørensen & Yamasaki, Zoologischer Anzeiger, 2024) and Campyloderes (1, C. vanhoeffeni Zelinka, 1913, nearly globally distributed).
ALLOMALORHAGIDA
(5:16/)122 spp. worldwide in two subclades: Pycnophyidae (8/101, MAP) and Anomoirhaga (4:8/21). Brazil includes at least four species in this group, in three families: Pycnophyes sp. and Kinorhynchus sp. in Pycnophyidae (Amaral et al., Biota Fapesp-Araçá, 2018), and two Anomoirhaga: Cateria styx Gerlach, 1956 (Cateriidae, M. Herranz et al., Zoologischer Anzeiger, 2019, also from Chile) and Franciscideres kalenesos Dal Zotto, Di Domenico, Garraffoni & Sørensen, 2013 (Franciscideridae, M. dal Zotto et al., Systematics and Biodiversidad, 2013 | also from E Argentina, Rucci et al., Zootaxa, 2020).
▉ LAST UPDATE OF KINORHYNCHA IN JANUARY/FEBRUARY 2026
24. PRIAPULIDA ‣ small marine group comprising (5:7/)23 spp. across four clades, three of which are successively basal (microscopic and meiofaunal) and one derived, macroscopic clade (Raeker, J. et al., Molecular Phylogenetics and Evolution, 2025). The work of van der Land (Zoologische Verhandelingen, 1970) is referenced here as A/1972.
All data presented below follow Catalogue of Life/Priapulida at the global level and CTFB for Brazil, with occasional additions highlighted in the text.
MEIOPRIAPULIDAE
Microscopic. Includes only Meiopriapulus fijiensis Morse, 1981, collected in Adaman islands in Bengal Bay, Fiji islands and Cheju island in South Korea (Sørensen, MV et al., Zoologischer Anzeiger, 2012).
TUBILUCHIDAE
Microscopic. 10 spp. worldwide in a single genus, Tubiluchus: T. corallicola (Panama, Curazao, Barbados, Bermuda, Bonaire, Bahamas, Florida), T. remanei (Egyptian coast of Red Sea, SEE), T. philippinensis (Mactan island near Cebu, C Philippines, SEE), T. australensis (Australian Great Barrier Reef), T. arcticus (White Sea and Barents Sea in Russia), T. vanuatuensis (New Hebrides island in Vanuatu), T. troglodytes (submarine caves in Apuglia, SE Italy), T. lemburgi (Canary Islands, Spain, and Cape Verde, ES), T. soyoae, T. pardosi (Kouri island near Okinawa in Ryukyu Islands, and Suruga Bay in SE Honshu, Japan, SEE), complemented by several reports of undetermined Tubiluchus specimens, e.g. from Playa Caletones in NE Cuba, Kos area in SW Turkiye (SEE), Hawaii, Koster area in SW Sweden and Cantabrian Sea in N Spain, by Schmidt-Rhaesa (Zoologischer Anzeiger, 2013 | 2017).
CHAETOSTEPHANIDAE
Microscopic, synonym of Maccabeidae. A single genus, Maccabeus, with two spp. from SE coast of Cyprus and Andaman Sea at 2,000 m deep (SEE | SEE).
MACROSCOPIC PRIAPULIDA
Two families and (4/)10 spp. of macroscopic Priapulids.
HALICRYPTIDAE ‣ a single genus, Halicryptus, and two species (Wikipedia | A/1972): H. higginsi Shirley & Storch, 1999 (coast of Alaska) and H. spinulosus von Siebold, 1849 (Labrador, coast of Alaska, E Greeland, Iceland, coasts of N Russia, N Scandinavia and Baltic Sea, A/1972).
PRIAPULIDAE ‣ (3/)8 spp. Some deep records was made in Pacific coast from NW Mexico to America Central, and around Java in Indonesia (A/1972), and lack specific definition.
Acanthopriapulus horridus Théel, 1911 (SE Brazil to Chile, Rhaesa et al., Zoologischer Anzeiger, 2022, known only from six specimens, three from Brazil).
Priapulopsis australis de Guerne, 1886 (New Zealand, South Africa, southern Chile and Argentina, A/1972).
Priapulopsis bicaudatus Danielssen, 1868 (NE U.S.A to Newoundland in Canada, around Greenland, North Sea to Severnaya Zemlya in Russia, A/1972).
Priapulopsis cnidephorus Salvini-Plawen, 1973 (incompletely described, questionable, Mediterranean Sea, SEE).
Priapulopsis papillatus Schmidt-Rhaesa & Raeker, 2025 (endemic to New Zealand, SEE).
Priapulus abyssorum Menzies, 1959 (off coast of Costa Rica, 5,680 m deep, SEE).
Priapulus caudatus Lamarck, 1816 (Alaska to California, Artic Alaska and Canada, over coasts of Greenland, NE U.S.A to Newoundland in Canada, Iceand to Chukotka in Russia, Kamchatka from Japan, A/1972).
Priapulus tuberculatospinosus Baird, 1868 (New Zealand, around Antartida, southern Chile and Argentina, A/1972).
▉ LAST UPDATE OF PRIAPULIDA IN JANUARY/FEBRUARY 2026
25. NEMATOMORPHA ‣ (3:21/)471 spp. worldwide (Catalogue of Life/Nematomorpha), parasitoid animals superficially similar to nematode worms in morphology, in two higher groups. Adult worms are free-living, but the larvae are parasitic on aquatic and terrestrial Arthropoda. (3:7/)23 in Brazil (CTFB/Nematomorpha).
NECTONEMATOIDA
5 marine species in a single genus, Nectonema: N. agile Verrill, 1879 (Atlantic Ocean and Mediterranean and Black Seas op to Brazil, Kakui et al., Parasitology Research, 202), N. melanocephalum Nierstrasz (Indonesia), N. munidae Brinkmann, 1930 (Belgium and Norway), N. svensksundi Bock, 1913 (Spitzbergen, Norway) and N. zealandica Poinar & Brockerhoff 2001 (New Zealand).
GORDIOIDA
(2:20/)466 spp. worldwide and (2:6/)22 in Brazil. Nine genera of both families in South America (CTFB | KNAF/2020), all these in Brazil except Gordionus (KNAF/2020). 77 spp. occur in Neotropical region (KNAF/2020).
▉ LAST UPDATE OF NEMATOMORPHA IN JANUARY/FEBRUARY 2026
26. NEMATODA ‣ roundworms show high homogeneity in their morphology and anatomy, with a transparent body, filiform, usually small in free living forms (length 0.2–3mm), pointed at one or both ends, covered by smooth cuticle, and without appendages. It is very
large group living in all habitats: terrestrial and aquatic (sea bottom and all kinds of freshwater),
and as parasites in plants, animals, and humans. Parasitic species living in cultivated plants or breeding animals are well
known whereas the free-living nematodes are still poorly recognized. Many free-living species are eurytopic — such species
can be found in different environments (terrestrial or aquatic) as well as in various climatic zones. They are very resistant to
unfavorable life conditions such as food and oxygen scarcity, and their presence was even discovered in paleometeoric waters
(3,000 – 12,000 years old) at the depth 0.9 – 3.6 km. These are first multicellular organisms found in such deep
subsurface of the Earth. Their eggs are resistant to desiccation and extreme temperatures so they can be easily transported for long distances by wind or water (White & Culver, Enciclopedia of Caves, 2019, 3rd edition).
(334:3,280/)23,627 spp. in two classes: Enoplea with (92:794/)7,706 spp. (Catalogue of Life/Enoplea) and Chromadorea with (242:2,486/)15,921 spp. (Catalogue of Life/Chromadorea). In Brazil occur (32:82/)179 spp. of Enoplea and (92:489/)1,385 in Chromadorea (CFTB/Nematoda), totaling (124:571/)1,564 spp.
Some checklists includes at least 151 genera of Nematoda in Gulf of Mexico (in 32 families, Beaton, KR et al, Journal of Experimental Marine Biology and Ecology, 2018), and a checklist of aquatic nematodes from Cuban Archipelago (469 spp., Pérez-Gracía, Zootaxa, 2020).
Possibly 8,000-9,000 Nematoda parasites Craniata, and lists of Brazilian parasitic nematodes are available for the following host groups: Actinopteri and alies fishes (Luque et al., Zootaxa, 2011 - 74/215 spp.), Amphibia (Vicente et al., Rev. Bras. Zool., 1990 - 14:24/63 spp.), reptiles (Vicente et al., Rev. Bras. Zool., 1993 - 23:55/121 spp.), Avialia (Vicente et al., Rev. Bras. Zool., 1995 - 23:75/257 spp.) and Mammalia (Vicente et al., Rev. Bras. Zool., 1997 - 45:160/495 spp.). For a excelent work of all 15 lineages of parasitic Nematoda - with hosts - see Blaxter & Koutsovoulos (Parasitology, 2015). Marine nematodes in Brazil, in April, 2017, includes (72:372/)450 spp. in 11 orders (Venekey, Zootaxa, 2017).
Placentonema gigantissima Gubanov, 1951 (Tetrameridae) is potentially the largest nematode worm ever described, with a length of 8.4 m and a diameter of 2.5 cm, discovered in the 1950s around the Kuril Islands, E Russia; this species develops its parasitic nature by utilizing nutrients found in the endometrium of female sperm whales and forming as spiriud (small, embroyonated) eggs; it can parasitize not only the placenta, but also the uterus, reproductive tract, mammary glands, or subdermis of the sperm whale (Wikipedia).
▉ LAST UPDATE OF NEMATODA IN JANUARY/FEBRUARY 2026
27. TARDIGRADA ‣ (33:162/)1,490 spp. worldwide (Catalogue of Life Eutardigrada | Heterotardigrada | Mesotardigrada) across three classes: Mesotardigrada (1:1/1), Eutardigrada (17:82/921), and Heterotardigrada (15:79/568), in Jan 26, 2026. Brazil includes (7:20/)43 spp. of Eutardigrada and (12:29/)55 of Heterotardigrada (CTFB/Tardigrada).
Mesotardigrada is a monotypic class, endemic to eastern Kyushu Island in S Japan, however, the type material has been lost, and the class is now considered nomen dubium (Grothman, Zoological Science, 2017). Barros, R.C. (Arquivos de Zoologia, 2020) recognized 100 species of tardigrades in Brazil, of which 70 are terrestrial and 30 marine, distributed between Eutardigrada (3 orders: 20 families / 43 species) and Heterotardigrada (10 orders: 29 families / 57 species). Additionally, Kaczmarek et al. (Zootaxa, 2015) listed 197 marine tardigrade taxa, with 2,240 records from 39 oceans and seas around the world.
For South America diversity of non-marine Tardigrada, see Kaczmarek et al. (Zootaxa, 2015); following this source, Brazil has the third freshwater diversity in South America (20/61), being surpassed by Argentina (30/111) and Chile (21/63). Only eight species are endemic to Brazil (numbers 4, 24, 68, 139, 145, 181, 185 and 198 of this work), none belong to a endemic genus. Only the family Oreellidae in South America does not occurs in Brazil (SEE).
Mexico has only (43/)75 spp. documented (Flores-Romero et al. The European Zoological Journal, 2025) and Colombia has 29 (Daza, A et al., Anais da Academia Brasileira de Ciências, 2025).
TARDIGRADA UPDATED IN JAN 26, 2026
▉ LAST UPDATE OF TARDIGRADA IN JANUARY/FEBRUARY 2026
28. ONYCHOPHORA ‣ (54/)220 valid spp. worldwide (Onychophora Website) in two families, Peripatopsidae (41/135) and Peripatidae (13/82). None country has the two families simultaneously.
The main characteristics distin-guishing the two taxa include (1) the position of the genital opening between the last (Peripatopsidae) or pen-ultimate leg pair (Peripatidae), (2) the presence (Peripatidae) or absence of a diastema (Peripatopsidae) on theinner blades of the jaws, (3) the structure of the primary dermal papillae with (Peripatidae) or without a con-striction (Peripatopsidae), (4) the solubility (Peripatidae) or dissolubility of body pigments (Peripatopsidae) in ethanol, and (5) the number of leg pairs ranging from 13 to 29 in Peripatopsidae and from 19 to 43 in Peripatidae (Mayer, Zootaxa, 2007).
PERIPATIDAE
(13/)82 spp. in Cerradopatus (1), Eoperipatus (4), Epiperipatus (30), Heteroperipatus (2), Macroperipatus (4), Mesoperipatus (1), Mongeperipatus (2), Oroperipatus (18), Peripatus (16), Plicatoperipatus (1), Principapillatus (1), Speleoperipatus (1), and Typhloperipatus (1).
Eoperipatus (4) occurs only in Malaysia (continental and part of Borneo), extreme SE Thailand and C & S Vietnan, and is unknown in Indonesia (SEE). Typhloperipatus (1) is endemic to Arunachal Pradesh state in NE India, recently collected after 111 years (SEE). Mesoperipatus (1) is endemic to W Gabon, in a small area in Ogoou´e River Basin (SEE). Speleoperipatus and Plicatoperipatus are endemic to Jamaica. Mongeperipatus is endemic to Costa Rica. Heteroperipatus occurs disjunctly in Panama and El Salvador. All remaining 4 genera occur in South America and in Brazil.
With the currently described species and the above circumscription, the three greatest diversities in genera among New World countries are, individually, Costa Rica (5), Jamaica (5), Brazil (4), Colombia (3), and Panama (3). For species, the leading countries are Brazil (22, additional but different data in Zoologia, 2009), Colombia (12), and Costa Rica (8). In numbers: Costa Rica (5/8), Jamaica (5/5), Brazil (4/22), Colombia (3/12), Panama (3/7), Trinidad (2/7), Venezuela (2/7), Haiti (2/6), Ecuador (1/6), Peru (1/5), Bolivia (1/3), Surinam (1/3), French Guiana (1/2), Mexico (1/1), El Salvador (1/1), Puerto Rico (1/1), Virgin Islands (1/1), Dominica (1/1), São Vicente (1/1), Barbados (1/1), Guatemala (1/1) and Guyana (1/1). Brazil includes species in Oroperipatus (2, see Contreras-Felix, Revista Mexicana de Biodiversidad, 2018 | C. S. Costa et al., Zoologia, 2018), Cerradopatus (1), Peripatus (2) and Epiperipatus (17).
Important changes can come from the information already pre-released from G. Giribet et al. (Invertebrate Systematics, 2018). However, almost all definitions about this family in unclear; works shows five distinct lineages: Asian members; Gabon member; Oroperipatus; Colombian-Ecuador lineage; remaining Neotropical taxa (Baker et al., Mol. Biol. Evol., 2021). Mongeperipatus, assignated as a new genus for Costa Rica (Barquero-González, Revista de Biologia Tropical, 2020), is strongly discrepant against Neopatida phylogeny (Barquero-González, Revista de Biologia Tropical, 2020).
PERIPATOPSIDAE
(41/)138 spp. (135 valids and 3 nomina nuda), with almost all genera endemic to explosive radiation in Australia (34/74) — except species in Chile (2/2: Metaperipatus and Parospisthopatus, both endemic), South Africa (2/34, Peripatopsis and Opisthopatus, both endemic), New Guinea and Maluku achipelago in Indonesia (1/6, Paraperipatus, exclusive), New Zealand (1/3, Peripatoides, endemic) and one genus Ooperipatellus (8), in Australia (6) and New Zealand (1), unique maong two distinctly areas.
▉ LAST UPDATE OF ONYCHOPHORA IN JANUARY/FEBRUARY 2026
29. ARTHROPODA ‣ 17 classes. Our extensive research identified 104,885 spp. of Arthropoda in Brazil, in 19,661 genera within 1,584 families. For taxonomy of Crustacean lineages, see Bernot, J.P. et al. (Molecular Biology and Evolution, 2023). For endemic families we follows Intreasures (SEE).
PHYLOGENY OF LIVING ARTHROPODA, BASED ON WIKIPEDIA (SEE) and Bernot, J.P. ET AL. (MOLECULAR BIOLOGY AND EVOLUTION, 2023)
29.1 PYCNOGONID CLASS ‣ a single order, Pantopoda, with (11:108/)1,476 spp. worldwide (Catalogue of Life). Brazil has (11:19/)74 spp. in Brazil (CTFB/Pycnogonida). A book covering all species of Brazil, with numerous images, is available from Editora UFPB. Mexico has (10:19/)58 spp. (Boletín de Investigaciones Marinas y Costeras, 2022).
Endoparasitism of Pycnogonida in Hydrozoa is rare, reported from Argentina, Australia, Germany, New Zealand, Papua New Guinea, South Africa, USA and Brazil, with two records on the coast of Paraná state (Bettim, A.L. & Haddad, M.A., Biota Neotropica, 2013), Anoplodactylus stictus Marcus, 1940 on hydroid species of Podocoryna (Hydractiniidae).
29.2 ARACHNIDA CLASS ‣ (859:13,536/)110,203 spp. in 17 orders (Opilioacarida, Holothryda, Ixodida, Mesostigmata, Trombidiformes, Sarcoptiformes, Pseudoscorpionida, Opiliones, Xiphosura, Palpigradi, Solifugae, Ricinulei, Scorpionida, Araneae, Amblypygi, Schizomida, Thelyphonida), 16 of these in Brazil (excludes Merostomata). Mexico no includes Holothryda.
Based on Wikipedia (SEE), Arachnida can be divided in five main taxa: Acaromorpha (Parasitiformes [Opilioacarida, Holothryda, Ixodida, Mesostigmata], Acariformes [Trombidiformes, Sarcoptiformes] and Pseudoscorpiones), Opilionomorpha (Opiliones), Solifugomorpha (Palpigradi, Solifugae, Ricinulei and Xiphosura), Scorpionomorpha (Scorpiones) and Pantetrapulmonata (Araneae, Amblypygi, Schizomida and Thelyphonida). Detailed data on the six orders of mites and acari in Brazil are available in Jacinavicius, F. C. et al. (Zoologia, 2025).
Among marine Acari, with near 900 spp. worldwide, only 10 spp. occur in Brazil, in four families: Halacaridae (Copidognathus, Agauopsis, Agaue), Tydeidae (Tydeus), Selenoribatidae (Schusteria) and Hyadesiidae (Amhyadesia), in Pepato & Tiago (Biota Neotropica, 2003). In South American records of freshwater Halacaridae includes 9 ssp. in Lobohalacarus-3, Peregrinacarus-1, Soldanellonyx-2, Limnohalacarus-2, and Porohalacarus-1, in Argentina, Brazil, Chile, Peru and Venezuela — and 2/3 in Mexico (Ojeda & Herrera-Mares, Entomological Communications, 2022).
ACAROMORPHA
ORDER OPILIOACARIDA
A single family worldwide (Opilioacaridae), with (14/)59 spp. (Catalogue of Life) collected in New World, Italy, Greece, Algeria, tropical Africa, Madagascar, Yemen, Kazakhstan, India, Thailand and Australia. (4/)25 spp. occur in New World (Berbardi & Borges-Filho, Subterranean Biology, 2018). Brazil has the highest known diversity to date, with (4/)18 spp. (CTFB/Opilioacarida): Brasilacarus and Amazonacarus are endemic to the country, while Neocarus and Caribeacarus also occur in other regions (Bernardi et al., Subterranean Biology, 2020). Mexico has (2/)8 spp. (M. Perez et al., Revista Mexicana de Biodiversidad, vol. 85, 2014).
ORDER HOLOTHRYDA
(3:15/)31 spp. (Catalogue of Life). Allothyridae and Holothyridae do not occur in the New World. New World family Neothyridae has six spp. in three genera: Neothyrus ana Lehtinen, 1981, from Peru, Neothyrus sp. from Venezuela, Diplothyrus schubarti Lehtinen, 1999 and D. lehtineni Vázquez, Araújo & Feres, 2016 from Brazil (CTFB/Holothryda), D. lecorrei Klompen, 2010 from French Guiana, and Caribothyrus barbatus Kontschán et Mahunka, 2004 from República Dominicana (Bernardi L. F. O. et al., Acarologia, 2011 | Vázquez M.M. et al., Acarologia, 2016).
ORDER IXODIDA
(3:60/)902 spp. Nuttalliellidae (1/2) is restricted to Africa. In Brazil there (2:9/)78 spp. documented (CTFB/Ixodida | Dantas-Torres et al., Science Direct, 2019, assignated here as DT/2019). World checklist of all Ixodida is available in S. C. Barker & A. Murrell (BOOK, 2008). Mexico has (2:10/)100 spp. in this order (M. Perez et al., Revista Mexicana de Biodiversidad, vol. 85, 2014).
ARGASIDAE ‣ (13/)215 spp. in World (Wikipedia), (4/)24 spp. in Brazil (CTFB), 18 of them belong to Ornithodorus. Otobius megnini Dugès, 1883, is an economically important soft tick as it parasitizes livestock mostly cattle, goats, sheep, and horses and also infests humans. Its original center of distribution is considered to be the SW North America from where it spread to Central and South America (Rajakaruna & Diyes, Ticks and Tick-Borne Pathogens, 2018), and considered non-native to Brazil.
IXODIDAE ‣ (46/)785 spp. worldwide (Catalogue of Life | Wikipedia), (5/)54 spp. in Brazil (CTFB), 35 in Amblyomma.
ORDER MESOSTIGMATA
(136:1,125/)7,669 spp. (Catalogue of Life), mainly free living mites. Laelapidae is recognized as the most diverse mesostigmatic family, with over (90/)1,300 spp. described worldwide (Nemati et al., Persian J. Acarol., 2021). Brazil has (56:217/)997 spp. (CTFB/Mesostigmata). Mexico has (50:158/)507 spp. in this order (M. Perez et al., Revista Mexicana de Biodiversidad, vol. 85, 2014).
■ endemic families in New World: Antennochelidae (1/1, Costa Rica) and Costacaridae (1/1, Mexico).
ORDER TROMBIDIFORMES
(164:2,638/)17,389 spp. (Catalogue of Life). Brazil has (78:435/)1,435 spp. (CTFB/Trombidiformes). Mexico has (78:328/)1,208 spp. in this order (M. Perez et al., Revista Mexicana de Biodiversidad, vol. 85, 2014).
■ endemic families in New World: Amoenacaridae (1/3, USA), Amphotrombiidae (1/1, USA), Crotalomorphidae (1/1, USA).
ORDER SARCOPTIFORMES
(270:2,487/)14,064 spp. (Catalogue of Life) in three groups: Astigmata (placed here within Oribatida), Endeostigmata and Oribatida. Brazil has (139:456/)1,118 spp. (CTFB/Sarcoptiformes). Mexico has (154:402/)801 spp. in this order (M. Perez et al., Revista Mexicana de Biodiversidad, vol. 85, 2014).
■ endemic families in New World: Tubulozetidae (1/1, Ecuador), Brazilobatidae (1/1, Brazil), Elliptochthoniidae (1/9, USA), Proteonematalycidae (1/1, USA).
ORDER PSEUDOSCORPIONA
(26:477/)4,350 spp. worldwide (WAC) — USA (433 spp.), Spain (286), Italy (276), China (257), Australia (245), Brazil (186), Mexico (175), South Africa (166) and India (164) leads worldwide (distribution browse).
(15:66/)189 in Brazil (LIST): Amblyolpiidae (1/1), Atemnidae (4/5), Bochicidae (1/4), Cheiridiidae (2/4), Cheliferidae (4/5), Chernetidae (24/71), Chthoniidae (10/37), Feaellidae (1/1), Geogarypidae (1/5), Hesperolpiidae (3/13), Ideoroncidae (2/11), Olpiidae (2/3), Pseudochiridiidae (1/1), Syarinidae (4/10), and Withiidae (6/18). A notable record is the family Feaellidae, which includes three genera with 13 spp., and the monotypic Iporangella from Alto do Ribeira area in São Paulo state (Harvey et al., Journal of Arachnology, 2016).
Mexican families Neobisiidae, Menthidae and Stemophoridae do not occur in Brazil. For images of species from all families, see Pseudoscorpions of the World/Families.
OPILIONOMORPHA
ORDER OPILIONES
(72:1,721/)6,888 spp. in 4 living suborders (Catalogue of Life), all of which are represented in Brazil: Cyphophthalmi (7:51/257, three families in New World), Eupnoi (8:265/1,853), Dyspnoi (11:54/429, northern Hemisphere to Mexico and Thailand, Australia, South Africa, also Brazil to Chile, SEE | SEE) and Laniatores (45:1,348/4,347). For a global diversity overview of Opiliones by country, see Checklist of valid genera of Opiliones of the World (by Adriano Kury). Brazil has (19:304/)1,008 spp. (960 endemic), the highest diversity worldwide (CTFB/Opiliones), followed by Venezuela (380), Indonesia (341), and the USA (302) worldwide (Kury et al., Zootaxa, 2025).
■ endemic families in New World: Otilioleptidae (1/1, Argentina) and Cryptomastridae (2/4, USA).
SOLIFUGOMORPHA
ORDER XIPHOSURA
4 living spp. in the family Limulidae (Vestbo et al, Frontiers in Marine Science, 2018): Carcinoscorpius rotundicauda Latreille, 1802 (E India to S Vietnam, SE China, and some places in Java, Borneo, Sumatra, SW New Guinea and the Philippines), Limulus polyphemus L., 1758 (Atlantic coast of USA from Maine to Louisiana, and Yucatan Peninsula in Mexico, MAP), Tachypleus gigas O. F. Müller, 1785 (S India to E Vietnam, and some places in Java, Borneo, Sumatra, Sulawesi and the Philippines), and T. tridentatus Leach, 1819 (S India to W Thailand, Cingapura, S Vietnam to S Japan and Korea, and Java, Borneo and Palawan in the Philippines).
ORDER PALPIGRADA
(2:6/)138 spp. worldwide (WAC), (1:3/)24 in Brazil (LIST), followed by Madagascar (2:3/19), Italy (18), Spain (12) and France (11) in diversity of species worldwide.
EUKOENENIIDAE ‣ comprises four genera: Allokoenenia (3) from Guinea (1) and Brazil (2, Bahia and Pará states, Souza M.F.V.R & Ferreira R.L, European Journal of Taxonomy, 2022), Eukoenenia (115), cosmopolitan, with 20 spp. recorded in Brazil (Sousa & Ferreira, Zootaxa, 2020), Koenenioides (8) from E Africa to SE Asia and China, and Leptokoenenia (5) in Saudi Arabia (1), Italy (1), Congo (1) and in two caves from SE Pará state in N Brazil (2).
PROKOENENIIDAE ‣ two genera: Prokoenenia (7) in Chile, China (SEE) and Thailand one each, and USA (Texas and California) and Indonesia two each; and Triadokoenenia, monotypic endemic to Madagascar.
ORDER SOLIFUGAE
(15:144/)1,213 spp. worldwide (WAC), in two groups: Boreosolifugae (5 families, only Eremobatidae in New World) and Australosolifugae (7 families, Ammotrechida, Mummuciidae and Daesiidae in New World), by Kulkarni, S.S. et al. (iScience, 2023). Kulkarni, S.S. et al. (Molecular Phylogenetics and Evolution, 2024) adds three new families (one Boreosolifugae and two Australosolifugae, none in New World), totaling 15 overall.
(2:6/)14 spp. in Brazil, ranking only 32nd in global diversity (LIST). USA (176 spp.), South Africa (163), Namibia (128), Mexico (83), Iran (70), Israel (65), Somalia (54) and Ethiopia (52) have the highest species diversity worldwide (distribution browse).
DAESIIDAE (30/216) ‣ three genera in New World, Ammotrechelis (1, Chile), Syndaesia (1, Argentina) and Valdesia (1, Argentina).
EREMOBATIDAE (8/194) ‣ from NW Canada to Honduras. All genera in USA (one endemic), seven of these extend to Mexico, and two reach Canada. Eremobates is the most diverse genus, with 97 spp.
MUMMUCIIDAE (8/80) ‣ exclusvely from South America. Based on WAC/Mummuciidae, Trujillo (Arthropod Systematics and Phylogeny, 2017), and and Souza, Ferreira & Carvalho (Zootaxa, 2021), eight genera belongs this family: four genera are national endemic in Argentina (Cordobugida, Curanahuel, both monotypic), Peru (Vempyroniellla) and Paraguay (Mummucipes), and four are widely distributed: Mummucina (6, Ecuador, Peru, Chile), Uspallata (1, from Argentina, Chile, Peru and Brazil), Mummucia (8, Brazil/2, Peru, Bolivia, Argentina, Paraguay, Chile, Uruguay) and Gaucha (13, Brazil/10, Argentina, Bolivia, Uruguay). Brazil has (4/)13 spp. and Argentina has (5/)8 spp.
Vempironiella aguilari Botero-Trujillo, 2016, endemic to coast of Lima regin, Peru, is the smallest solifugae worldwide, with males measuring 3.90–5.85 mm in total body length, with the second smallest being the southern African Lawrencega minuta Wharton, 1981 whose males measure 5–8 mm (Trujillo, Journal of Arachnology, 2016).
AMMOTRECHIDAE (26/100) ‣ , exclusive New World. Based on Botero-Trujillo (Arthropod Systematics and Phyogeny, 2023), five subfamilies, three of these subfamilies contain only a single genus endemic to Argentina (SEE), and 17 genera in New World, all in South America, 10 national endemic and 7 are widely distributed. Endemic genera in Argentina (6, Procleobis, Titanopuga, Notopuga, Oltacola, Dasycleobis and Cuyanopuga), Ecuador (Campostrecha), Chile (Chileotrecha and Sedna) and Peru (Chinchippus). Widely genera includes Saronomus (1, Colombia and Venezuela), Eutrecha (3, Colombia and Venezuela), Xenotrecha (1, Brazil and Venezuela), Pseudocleobis (21, Peru, Bolivia, Chile and Argentina), Ammotrecha (10, USA to Costa Rica, Brazil and Chile one endemic each), Ammotrechella (15, USA to Panama, Caribbean, one extending to Ecuador and Venezuela) and Ammotrechula (USA, Mexico, America Central, one in Colombia and Ecuador). Argentina has (7/)24 spp., followed by Chile (4/8), Colombia (4/5), Venezuela (4/4), Peru (3/9), Ecuador (3/3), Brazil (2/2) and Bolivia (1/4).
ORDER RICINULEI
(3/)104 spp. worldwide (WAC), all in Ricinoididae: Ricinoides (16, W Africa), Cryptocellus (47, 14 from Nicaragua to Panama, and the remaining 33 from Suriname to Peru and N Brazil) and Pseudocellus (41, Texas to Panama and Caribbean, mainly in Mexico and Cuba). Mexico (1/21), Brazil (1/13, LIST), Cuba (1/12) and Colombia (1/10) have the highest species diversity worldwide (distribution browse).
Molecular and morphological data published by S. Sato et al. (Molecular Phylogenetics and Evolution, 2024) suggest the existence of 3 to 5 lineages consistent with genus level in Ricinulei: Ricinoides (basal, Africa), rogue Pseudocellus (Mexico) + Cryptocellus, and Cryptocellus magnus group (Andes) + Pseudocellus s.s. However, the new lineages suggested as valid genera have not yet been formalized.
SCORPIONOMORPHA
ORDER SCORPIONA (THE SCORPION FILES)
(24:235/)2,913 spp. (The Scorpion Files Newsblog). 10 families occur only in Old World: Akravidae (1/1, Israel), Belisariidae (2/3, France, Spain, Italy), Chaerilidae (1/54, Asia), Hemiscorpiidae (1/17, Middle East and eastern parts of Asia), Heteroscorpionidae (1/6, Madagascar), Iuridae (7/14, Turkey, Iraq, Syria, Greece), Pseudochactidae (5/7, Tajikistan, Uzbekistan, Afeganisthan, China, Laos and Vietnan), Rugodentidae (1/1, India), Scorpionidae (18/196, Old World) and Scorpiopidae (2/108, Asia).
Five occur only in North America up to Guatemala zone: Anuroctonidae (1/1, USA to NW Mexico), Hadruridae (2/9, USA and N Mexico), Superstitioniidae (1/1, USA and N Mexico), Typhlochactidae (4/11, endemic to E & C Mexico) and Vaejovidae (25/236, SW Canada to Guatemala).
Five families are endemic to South America or widely in southern Hemisphere: Anatheridae (7/128, mainly distributed in South America, also in Asia and Africa), Caraboctonidae (2/26, Galapagos, mainland Ecuador, Peru and Chile), Hormuridae (11/115, Caribbean to South America, SE Asia to Australia), Troglotayosicidae (1/6, S Colombia and Ecuador) and Bothriuridae (17/166, mainly Ecuador to Argentina and Brazil, also Lisposoma and Brandbergia in Namibia, and Cercophonia in Australia).
Four families are widely distyributed worldwide: Buthidae (100/1,370, worldwide), Chactidae (14/209, North to South America, Nullibrotheas allenii Wood, 1863 is the only representative in the Nearctic region), Euscorpiidae (6/109, disjunct in Mexico, Guatemala and Mediterranean region) and Diplocentridae (10/140, Mexico, SW USA, Belize, Costa Rica, El Salvador, Guatemala, Honduras, Nicaragua, Colombia, Venezuela, Greater & Lesser Antilles, Egypt, Iran, Israel, Jordan, Lebanon, Oman, Saudi Arabia, Syria, Yemen).
BRAZIL
Joined data for LEE (2023, SEE) and some updates, Brazil has the third highest diversity worldwide, with (5:25/)171 native spp. in Ananteridae (1/31, Ananteris-31), Buthidae (8/79, Ischnotelson-2, Jaguajir-3, Microtityus-2/SEE, Physoctonus-2, Rhopalurus-1, Tityus-66, Troglorhopalurus-2, Zabius-1), Chactidae (11/46, Auyantepuia-5, Broteochactas-6, Brotheas-10, Chactas-2, Chactopsis-5, Chactopsoides-1, Guyanochactas-2, Hadrurochactas-5, Neochactas-3, Teuthraustes-5 and Vachoniochactas-2), Bothriuridae (4/22, Bothriurus-17, Brachistosternus-1, Thestylus-3, Urophonius-1) and Hormuridae (1/3, Opisthacanthus-3), behind Mexico (9:38/281) and Venezuela (4:/230, being 128 Buthidae, 97 Chactidae, 2 Hormuridae and 3 Diplocentridae), and ahead of India (114), USA (111) and South Africa (104).
MEXICO/SOUTH AMERICA
Three families of scorpions occur in South America but have no representatives in Brazil: Troglotayosicidae (exclusive), Caraboctonidae (exclusive) and Diplocentridae (in South America represented by a restricted genus, Tarsoporosus, with 5 spp. in Colombia and Venezuela). (5:15/)82 spp. in Colombia (SEE).
Mexico has (9:38/)281 spp. (Santibáñez-Lápez et al., Toxins (Besel), 2016) in Buthidae (2/44), Hadruridae (2/9, in Mexico all genera and species), Chactidae (1/1), Diplocentridae (3/58), Euscorpiidae (3/8), Superstitioniidae (1/1), Anuroctonidae (1/1), Typhlochactidae (4/11) and Vaejovidae (21/149).
Brazilian yellow scorpion Tityus serrulatus Lutz & Mello, 1922 (Buthidae), widely distributed but endemic to country, is one of most poisonous in the world, and the worst in the New World (Fatal Stingers | Planet Deadly — details in Cologna et al, Protein & Peptide Letters, 2009). Microtityus adriki Moreno-González, Bertani & Carvalho, 2024 (Buthidae), endemic to Roraima state, is the smallest scorpion of Brazil, mensuring 12.39-19.47 mm (Moreno-González et al., Zoosystema, 2024). Tityus achilles Laborieux, endemic to C Colombia (Cundiramarca), 2024 in the first toxungen-spraying scorpion in South America (Laboriuex, ZJLS, 2024).
■ endemic families in New World: Typhlochactidae (4/10, Mexico).
PANTETRAPULMONATA
ORDER ARANEAE
(139:4,502/)53,686 spp. worldwide (World Spider Catalogue). Worldwide highest diversities are China (5,476), Australia (4,034), USA (3,558), Brazil (79:638/3,248, CTFB/Araneae), Russia (2,497), Mexico (66:534/2,295, SEE) and South Africa (2,253), by Zamani et al.(Diversity, 2023).
There are three maing groups of spiders (Wikipedia): Mesothelae has a single family, Liphistiidae, with (8/)194 spp. from Japan to Sumatra, and two clades widely worldwide. 97 families in New World.
TAXONOMY
MYGALOMORPHA
32 families, 13 in Brazil (highest family-diversity in New World: Actinopodidae, Barychelidae, Cyrtaucheniidae, Dipluridae, Halonoproctidae/Wikipedia, Idiopidae, Ischnothelidae, Mecicobothriidae, Microstigmatidae, Paratropididae, Pycnothelidae, Rhytidicolidae, and Theraphosidae).
19 families do not occur in Brazil, 8 only Old World: Macrothelidae (Europe to C Africa, E Asia), Ctenizidae (France, Italy, Greece, Turkyie), Bemmeridae (S Africa, S & SE Asia), Entypesidae (S Africa), Stasimopidae (South Africa), Anamidae (Australia), Atracidae (Australia), and Porrhothelidae (New Zealand). 11 occur in New World: Antrodiaetidae (North America, Japan), Nemesiidae (USA to Mexico, Magreb to N China and Vietnam, Mozambique), Atypidae (North America, Asia, Africa, Europe), Microhexuridae (Canada, USA), Megahexuridae (W USA), Hexurellidae (USA, Mexico), Euagridae (USA to America Central, southern South America, southern Africa, Asia, Oceania), Euctenizidae (USA to Mexico, Guadalupe), Hexathelidae (Argentina, Chile, Australia and New Zealand), Melloinidae (Panama to Venezuela), and Migidae (Argentina, Chile, Africa, Oceania).
ARANEOMORPHA
106 families, 66 in Brazil: Agelenidae, Amaurobiidae, Anapidae, Anyphaenidae, Araneidae, Caponiidae, Cheiracanthiidae, Cithaeronidae, Clubionidae, Corinnidae, Ctenidae, Deinopidae, Desidae, Dictynidae, Diguetidae, Drymusidae, Dysderidae, Eresidae, Filistatidae, Gallieniellidae, Gnaphosidae, Hahniidae, Hersiliidae, Linyphiidae, Lycosidae, Macrobunidae, Mimetidae, Miturgidae, Mysmenidae, Nesticidae, Ochyroceratidae, Oecobiidae, Oonopidae, Orsolobidae, Oxyopidae, Palpimanidae, Philodromidae, Pholcidae, Pisauridae, Prodidomidae, Salticidae, Scytodidae, Segestriidae, Selenopidae, Senoculidae, Sicariidae, Sparassidae, Symphytognathidae, Synotaxidae, Tetrablemmidae, Tetragnathidae, Theridiidae, Theridiosomatidae, Thomisidae, Titanoecidae, Trachelidae, Trechaleidae, Trochanteriidae, Uloboridae, Zodariidae, and Zoropsidae cited in CTFB/Araneae, plus Ancylometidae (Wikipedia | CTFB/Ctenidae), Dolomedidae (Wikipedia | CTFB/Pisauridae), Telemidae (a described species, Pinelema elinae Brescovit, Gallão & Cizauskas, 2023/SEE, and a unnamed species, also in Bahia state, SEE), Viridasiidae (Wikipedia | CTFB: Ctenidae), and Xenoctenidae (Wikipedia | CTFB: Ctenidae).
40 families absent in Brazil: 21 unknown in New World: 10 confined to zone from Indonesia to New Zealand (Cycloctenidae, Trachycosmidae, Stiphidiidae, Lamponidae, Nicodamidae, Periegopidae, Gradungulidae, Megadictynidae, Huttoniidae, Arkyidae), Fonteferreidae (Portugal), Archaeidae (South Africa, Madagascar and Australia), Synaphridae (Europe to C Asia, Madagascar), Penestomidae (South Africa, Lesotho), Udubidae (Africa, Madagascar, Sri Lanka), Phyxelididae (Africa, Turkyie, Indonesia), Stenochilidae (Iraq, C Asia to New Guinea), Psilodercidae (E Asia), Pacullidae (China to New Guinea), Psechridae (China to Australia); and 19 in New World: Argyronetidae (cosmopolitan except South America and tropical Africa), Cicurinidae (northern Hemisphere to Mexico and Vietnam), Pimoidae (North America, Europe and Asia), Lathyidae (worldwide except South America, Australasia and Oceania), Liocranidae (Canada to Peru, Argentina, Old World), Phrurolithidae (Canada to Mexico, Europe to Australia), Hypochiidae (North America, Mexico and E Asia), Homalonychidae (USA, Mexico), Trogloraptoridae (USA), Myrmecicultoridae (USA, Mexico), Archoleptonetidae (USA, Mexico, Guatemala, Panama), Leptonetidae (USA to Panama, Mediterranean region, E Asia), Plectreuridae (USA to America Central and Cuba), Cyatholipidae (Africa, Madagascar and New Zealand, Jamaica), Cybaeidae (northern Hemisphere, Sumatra, Colombia, Venezuela and Peru), Austrochilidae (Chile, Argentina and Tasmania), Malkaridae (Australia, Chile and Argentina), Mecysmaucheniidae (New Zealand, Chile and Argentina), and Physoglenidae (Australia, Chile).
Mexico has 61 families and USA has 60 among this clade.
NOTES
In Brazil, following data in Castanheira et al. (Biodiversity Data Journal, 2016), there are 875 spp. in São Paulo state and 808 in Rio Grande do Sul state. Nogueira et al. (CheckList, 2014) lists 529 spp. (many only morphospecies and undescribed) of spiders was found in Mount Neblina.
THERAPHOSIDAE
Among Theraphosidae (163/1071), notable centers of diversity include Brazil (47/202), Mexico (17/99), and Colombia (22/43). In Mexico, all species belong to Theraphosinae, except for Psalmopoeus victori Mendoza, 2014. The country hosts the largest endemic genera: Hemirrhagus (27) and Bonnetina (16). The USA has 29 species, all in the genus Aphonopelma.
Six subfamilies are not recorded from from the New World: Eumenophorinae (54, Sub-Saharan Africa, southern Arabian Peninsula, Madagascar, and India), Harpactirinae (59, widespread in the Old World), Ornithoctoninae (26, SE Asia), Poecilotheriinae (1/15, India and Sri Lanka), Selenocosmiinae (11/127, SE Asia to Australia), Stromatopelminae (3/9, Africa), Selenogyrinae (3/9, Africa, India, and Sri Lanka), and Thrigmopoeinae (1/9, S & E Asia).
Five subfamilies occur in New World: Psalmopoeinae (5/32 | all genera in Brazil, none endemic), Schismatothelinae (5/33 | 3 genera in Brazil, Sickius endemic, two from Panama to Peru, east up to T.Tobago), Ischnocolinae (17/108 | five genera exclusively in Old World, 12 in tropical America, five in Brazil, with the highest generic diversity, none endemic | national or locally endemic genera in India/2, Seychelles/1, Belize/1 and Hispaniola/1), Aviculariinae (7/31, all genera in Brazil except Antillena from República Dominicana, being four endemic) and Theraphosinae (43/553 spp., 26 genera in Brazil, highest generic-diversity | Brazil e Mexico 7 endemic genera each).
RECORDS
Atrax robustus complex (3, Atracidae) are iconic Australian species and considered among the most dangerously venomous spiders for humans (Novataxa). Theraphosa blondi Latreille, 1804 is found in northern South America, it is the largest spider in the world by mass (175 g) and body length (up to 13 cm), and second to the Heteropoda maxima Jäger, 2001 (Sparassidae, endemic to Laos) by leg span (Wikipedia).
■ endemic families in New World: Megahexuridae (1/1, USA) and Trogloraptoridae (1/1, USA).
ORDER AMBLYPYGI
(5:18/)282 spp. worldwide (WAC), only Charontidae (1/7, SE Asia and N Australia) not recorded in New World. Brazil (4/54, LIST) and Mexico (3/30) has the highest species diversity worldwide (distribution browse).
CHARINIDAE ‣ (4/)152 spp. among in Catageus (8, Asia), Weygoldtia (4, in Camboja, Laos and Vietnam), Sarax (38, Italy to Seychelles and Palau) and Charinus (102, widely distributed, 45 in Brazil). Charinus giganteus from N Bahia state, NE Brazil, is the largest species of genus, with 16mm adult form (Sousa & Ferreira, Zootaxa, 2025).
PARACHARONTIDAE ‣ two monotypic genera, Paracharon from Guinea Bissau and Jorottui endemic to N Colombia (American Museum Novitates, 2023).
PHRYCHIDAE ‣ 7 genera, six from Africa to SE Asia and Trichodamon (1) in E Brazil (Bahia, Goiás and Minas Gerais states).
PHRYNIDAE ‣ (6/)86 spp. in Acanthophrynus (1, Mexico and USA), Heterophrynus (19, N & C South America, 6 in Brazil), Paraphrynus (22, USA to Ecuador, mainly in Mexico, SEE) and Phrynus (42, USA to northern South America, one in Brazil, one also in Africa).
ORDER SCHIZOMIDA
(2:71/)377 spp. worldwide (WAC). Australia (74), Mexico (16/60), Cuba (14/57) and Brazil (6/19) have the highest species diversity worldwide.
HUBBARDIIDAE ‣ 42 genera in New World, 31 national endemic in Cuba (11, Cokendolpherius-2, Cubacanthozomus-1, Cubazomus-2, Dumitrescoella-1, Guanazomus-1, Heterocubazomus-1, Pinero-1, Reddellzomus-1, Siguanesiotes-1, Troglocubazomus-1, Vinabayesius-3), Mexico (7, Ambulantactus-3, Baalrog-4, Nahual-4, Olmecazomus-3, Pacal-3, Sotanostenochrus-2, Troglostenochrus-2, Schizophyxia-2), Brazil (3, Adisomus-1, Cangozomus-1, Naderiore-1), Colombia (2, Calima-5, Colombiazomus-2), Jamaica (2, Caribezomus-1, Stewartpeckius-1), Venezuela (3, Stenoschizomus-1, Wayuuzomus-1, Jipai-1/SEE), Puerto Rico (1, Luisarmasius-1), Ecuador (1, Tayos-1) and in USA (1, Hubbardia-9).
Harveyus (USA-1, Mexico-2) reaches from USA to Mexico. Two genera reaches from Mexico to America Central: Heteroschizomus (Mexico-2, Guatemala-2) and Mayazomus (Mexico-7, Guatemala-1). One genus is exclusive from Caribbean: Antillostenochrus (Cuba-9, Puerto Rico-1, Haiti-1, Dominican Republic-2). One genus reaches from Mexico to South America: Surazomus (Mexico-3, Costa Rica-7, Colombia-3, Ecuador-3, Peru-1, Bolivia-1, Brazil-9). Three genera reaches from America Central and/or Caribbean to South America: Hansenochrus (Martinique-1, Costa Rica-3, Panama-1, Venezuela-4, Guyana-2, Suriname-3, T.Tobago-3), Piaroa (Costa Rica-2, Panama-1, Colombia-6, Venezuela-2) and Rowlandius (Cuba-32, Puerto Rico-3, Haiti-1, Dominican Republic-12, Jamaica-4, Martinique-1, Costa Rica-2, Colombia-1, Venezuela-1, Brazil-6). Two genera are disjunct in E Asia and New World: Bamazomus (E Asia, Hawaii-1) and Schizomus (24, Asia to New Guinea-10, Africa-13 and Mexico-1).
Finally, Stenochrus is widely in New World (Mexico-7, Guatemala-2, Honduras-2, Nicaragua-2), with Stenochrus portoricensis the unique species of this genus in USA, Bermuda, Cuba, Puerto Rico, Dominican Republic, Jamaica, Dominica, Panama, Colombia, Ecuador and Brazil.
PROTOSCHIZOMIDAE ‣ (2/)16 spp., in Agastoschizomus (Mexico-7, USA-1) and Protoschizomus (8, Mexico).
ORDER THELYPHONIDA/UROPIGY
(17/)140 spp. (Wikipedia) in a single family, 11 genera occur in New World: Mastigoproctus (23, widely in tropical New World excluding Brazil, 10 in Mexico), Mimoscorpius (1, Guatemala), Mayacentrum (3, Guatemala, Belize, Honduras, El Salvador), Valeriophonus (1, Costa Rica), Ravilops (2, República Dominicana), Sheylayongium (1, Cuba), Wounaan (1, Colombia, SEE), Yekuana (1, Venezuela, SEE), Thelyphonellus (4, Colombia to Suriname and N Brazil/1), Amauromastigon (1, endemic to C & SE Brazil, Csiro) and Heptatarsus (2, endemic to C & SE Brazil, Csiro). (3/)8 spp. in Brazil.
29.3 CHILOPODA CLASS ‣ 5 orders and (17:445/)3,425 described species (according to the data gathered in the topics below). Outside Geophilomorpha, almost all families not recorded in Brazil are mutually disjunct in range. Brazil has (10:34/)148 spp. (CTFB). Mexico has (12:67/)162 spp. in Chilopoda.
Some checklist worldwide includes Geophilomorpha from Europe (37/179 spp., Bonato & Minelli, Zootaxa, 2014), Scutigeromorpha and Scolopendromorpha from Colombia (4:9/37 spp., Chagas-Jr et al, Zootaxa, 2014), and Lithobiomorpha from Austria, Germany, Switzerland, Scandinavia, the Netherlands, Belgium and northern and central France (Eason, Zoological Journal of the Linnean Society, 1982).
ORDER CRATEROSTIGMOMORPHA
Only two species in a single family, Craterostigmidae, known only from Tasmania (1) and New Zealand (1).
ORDER GEOPHILOMORPHA
7 families (Bonato et al., Cladistics, 2013 | Foddai et al., Amazoniana, 2000, for America Latina) and (265/)1,412 spp. (according to the data gathered in the topics below). (4:17/)70 spp. in Brazil, (28/)61 spp. in Mexico. For a lists of all Geophilomorpha genera in Neotropical region and all Geophilomorpha from Brazilian Amazon, see D. Foddai et al. (Anales del Instituto de Biología, 2004).
GEOPHILIDAE (including Aphilodontidae, Dignathodontidae, Linotaeniidae, Macronicophilidae and Tampiyidae: IRNMG | Bonato et al., Cladistics, 2013 | Foddai et al., Amazoniana, 2000 | Bonato & Ferreira, Organisms Diversity & Evolution, 2023 | Calvanese & Brescovit, Zootaxa, 2022 | Wikipedia/Mecophilus | Calvanese, Brescovit and Bonato, Zootaxa, 2019) ‣ (154/)753 spp. worldwide (Catalogue of Life).
NATIONAL ENDEMIC (23)
Cuba (Erithophilus-1), Pierto Rico (Portoricona-2), Mexico (Aztekophilus-2, Chomatophilus-3, Oligna-1, Pagotaenia-1), Panama (Barrophilus-1, Nabocodes-1), Peru (Peruphilus-1), Ecuador (Ecuadoron-1), Chile (Araucania-1, Chilenophilus-4, Filipponus-1, Ketampa-1, Nicopus-1, Orinomerium-1, Pandineum-10, Schizonium-6, Synerium-1), Brazil (Hyphydrophilus-1, Mairata-2, Mecophilus-3, Plutogeophilus-1).
POLINATIONAL ONLY NEW WORLD (15)
Tomotaenia (11, North America-10, Mexico-1), Garrina (13, USA, Mexico, Guatemala), Taiyuna (5, USA-4, Guyana-1), Gosipina (2, USA, Mexico), Sogona (5, USA-2, Mexico-2, Brazil-1), Nesidiphilus (7, SE USA, Nicaragua to Costa Rica, Caribbean), Polycricus (16, Mexico-11, Caribbean-1, Panama-1, Haiti-2, Chile-1), Apogeophilus (2, Caribbean-1, Argentina-1), Piestophilus (2, Honduras, Dominica), Suturodes (4, Guatemala-3, Honduras-1), Telocricus (5, Cuba-4, Haiti-1), Macronicophilus (4, Brazil-2, Ecuador-1 and Venezuela-1), Aphilodon (16, Argentina-3, Brazil-12, Paraguay-1), Dinogeophilus (2, Argentina, Uruguay), and Schendyloides (2, Argentina and Chile).
NEW AND OLD WORLD
Pachymerinus (9, Australia-3, Argentina-1, Chile-5), Pachymerium (38, 32 in Old World, Mexico-2, Cuba-2, Ecuador-1, Chile-1), Ribautia (45, Africa, Madagascar, New Caledonia, northern South America-22, 5 in Brazil), Eurytion (29, Africa, Australia, 8 in New World, in Peru, Argentina and Chile), Geoperingueyia (11, South Africa-10, Argentina-1), Schizonampa (3, Africa-2, Brazil-1), and Tuoba (10, Mediterranean, Cameroon, Ascension, Santa Helena, Seychelles, Japan to Vietnam, Australia, New Caledonia, Solomon Islands, New Zeland, California, Virgin Islands-1, Chile-2).
Brazil has (9/)28 spp., Mexico (10/)32. Highest diversities of genera in Chile (17), Mexico (10), Brazil (9) and Caribbean (6). Mecophilus sp. endemic to SE Brazil, are among the smallest centipedes in the order Geophilomorpha, reaching only 8 mm in length (Wikipedia).
GONIBREGMATIDAE (including Eucratonychidae, Neogeophilidae and Eriphantidae: IRNMG | Bonato et al., Cladistics, 2013 | Foddai et al., Amazoniana, 2000) ‣ (9/)20 spp. worldwide (Catalogue of Life), only three genera in America Latina: Evallogeophilus (1, endemic to Mexico), Neogeophilus (3, Mexico and Guatemala), and Eriphanthes (1, Baja California Sur in Mexico).
HIMANTARIIDAE ‣ (19/)72 spp. (Catalogue of Life), Macaronesia through the Mediterranean region and Middle Asia to India, known also from the Korean Peninsula and Japan, as well as from western part of North America to C Mexico (Ecologica Montenegrina, 2021). 10 spp. in America Latina, in Arcophilus (2, Mexico-1, Bolivia-1), Californiphilus (1, NW Mexico), Causerium (1, Mexico), Chomatobius (9, USA-5, Mexico-4), Geoballus (2, Mexico), and Straberax (1, Mexico).
MECISTOCEPHALIDAE (Foddai et al., Amazoniana, 2000) ‣ (11/)189 spp. worldwide (Catalogue of Life), only two genera in New World: Mecistocephalus (80, 5 in New World, USA, Panama, Caribbean, Brazil, Clipperton Island and Venezuela) and Tygarrup (15, Old World, one sp. in Guyana). (1/)2 spp. in Brazil.
ORYIDAE (Foddai et al., Amazoniana, 2000) ‣ (19/)47 spp. worldwide (Catalogue of Life), (7/)17 spp. in New World: Notiphilides (3, Mexico to South America, 1 in Brazil), Orphnaeus (20, tropical Africa, Asia and New World-6, 3 in Brazil), Titanophilus (4, Haiti, Peru, Venezuela), Pentorya (4, Africa, India and Venezuela-1), Heniorya (1, Brazil), Metaxythus (1, Chile) and Trematorya (1, Chile). The unique luminescent Chilopoda is member of this family, the pantropical Orphaneus brevilabiatus Newport, 1845 (Shimomura & Yampolsky, BOOK, 2019). (3/)5 spp. in Brazil, only one in Mexico.
SCHENDYLIDAE (including Ballophyidae: Bonato et al., Cladistics, 2013 | Foddai et al., Amazoniana, 2000 | Pereira, Papeis Avulsos de Zoologia, 2012) ‣ (50/)324 spp. worldwide (Catalogue of Life), 29 genera in America Latina: 19 are national endemics in Bahamas (Bimindyla-1), Puerto Rico (Algunguis-1, Clavophilus-1, Portoricellus-1), Mexico (Mexiconyx-1, Morunguis-1), Guatemala (Schendylellus-1, Sogodes-1, Sogolabis-1), Honduras (Tanophilus-1), Panama (Caritohallex-1, Cymochilus-1), T.Tobago (Leucolinum-1), Venezuela (Cerethmus-1, Koinethmus-1), Peru (Orygmadyla-1, Thindyla-1), Ecuador (Nannopodellus-1, Zygethmus-1); remainig are Schendylops (63, Africa, Madagascar, 51 in New World, 20 in Brazil), Ityphilus (23, pantropical, 18 in New World, 6 in Brazil), Pectiniunguis (23, North America to South America, Africa, Fiji, 7 in Brazil), Parunguis (4, California-1, Mexico-3), Marsikomerus (5, USA, Hawaii, Mexico-1), Nyctunguis (18, North America-15, Mexico-2, Peru-1), Diplethmus (6, Mexico to Peru), Ctenophilus (12, Africa-11, Haiti-1), Ballophilus (41, Asia to New Zealand, Puerto Rico-1, Peru-1, Argentina-1), and Taeniolinum (6, Panama-3, Caribbean-2, Brazil-1).
(4/)35 spp. in Brazil, (8/)14 in Mexico. Generic diversity by country: Mexico (8), Brazil (4), Panama (4), Peru (4), Guatemala (3), Puerto Rico (3), Colombia (2), Ecuador (2), Venezuela (2), Argentina (1), Caribbean (1), Peru (1), Puerto Rico (1), Costa Rica (1), Haiti (1), Bahamas (1), T.Tobago (1) and Honduras (1).
Schendylops oligopus Pereira, Minelli & Barbieri, 1995 and S. ramirezi Pereira, 2013, both endemic to Brazil, are therefore the unique representative of the order Geophilomorpha characterized by having 27 pairs of legs; the latter is one of the smallest members in this group of centipedes with 6-7 mm long (Pereira, L.A., Papéis Avulsos de Zoologia, 2013).
ZELANOPHILIDAE ‣ (3/)7 spp. in Tasmania and New Zealand.
ORDER LITHOBIOMORPHA
(122/)1,132 spp. described worldwide, all members with 15 trunk segments, in two families worldwide, marginally in tropical areas.
HENICOPODAE ‣ (22/)128 spp. worldwide (genera after Hollington & Edgecombe, Records of the Australian Museum, 2004 | Faralieva, Zalesskaja & Edgecombe, Arthropoda Selecta, 2004 | Shear, Zootaxa, 2018; species in Catalogue of Life/Henicopidae): Pleotarsobius (1, Hawaii), Speleopsobius (1, USA), Buethobius (5, USA), Yobius (1, USA), Zygethobius (5, North America), Rhodobius (1, Greece), Dzhungaria (Khazakhstan), Ghilaroviella (Tajikistan), Cermatobius (6, Indonesia, Japan, Kirghizia, China), Hedinobius (1, China), Shikokuobius (1, Japan), Anopsobiella (1, Vietnam), Analamyctes (2, Argentina), Catanopsobius (1, Chile), Anopsobius (11, Argentina, Chile, South Africa, Australia, New Zealand), Lamyctopristus (7, South Africa, Angola, Algeria), Remilamyctes (1, Madagascar), Dichelobius (2, Australia, New Caledonia), Easonobius (2, New Caledonia), Henicops (5, Australia, New Zealand and New Caledonia), Paralamyctes (Australia, New Zealand, southern South America, India, Madagascar, and South Africa) and Lamyctes (43, all continents except Antarctica and on many oceanic islands).
Paralamyctes includes Lamyctinus, Haasiella, Wailamyctes, Nothofagobius and Thingathinga, sometimes accepted as valid (RAM | Catalogue of Life | JNH).
For Lamyctes (unique genus in Brazil and Mexico), besides L. coeculus Brolemann, 1889, and L. emarginatus Newport, 1844, reported here as invasive species, few species occur in New World, namely in Peru (7), mainland USA and Hawaii (6), Chile (6), Mexico (2), Bolivia (2), Caribbean (2), Argentina (1) and Brazil (1, L. adisi Zalesskaja, 1994, known only from Amazonas state).
LITHOBIIDAE ‣ (100/)1,104 spp. (Catalogue of Life), unknown in Brazil, (25/)58 spp. in Mexico (Cupul-Magaña, BIOCYT, 2009): Arebius (2, SW USA to N Mexico), Arenobius (1), Atethobius (2), Cerrobius (1, Michoacan), Cruzobius (4, Vera Cruz), Delobius (4, DF, Puebla), Friobius (4, DF, Vera Cruz, Michoacan, Puebla), Garcibius (1, Nuevo Leon), Gosibius (1), Guerrobius (2), Labrobius (6, DF, Michoacan), Lithobius (6), Lobochaetotarsus (1), Malbius (1), Mayobius (4, Guerrero, Puebla, Morelos), Mexicobius (5, Puebla, DF, Morelos), Mexicotarsus (1), Neolithobius (1), Nuevobius (1), Popobius (1), Sotimpius (1), Tidabius (1), Tropobius (2, Vera Cruz), Uncobius (2, Puebla), and Vulcanbius (3, Michoacan).
ORDER SCOLOPENDROMORPHA
(5:39/)825 spp. worldwide. All members has 21 to 23 segments except 25 in a single species from Trinidad & Tobago (Chagas-Jr, Manelli & Edgecombe, Organisms Diversity & Evolution, 2022). (3:12/)73 spp. in Brazil.
CRYPTOPIDAE ‣ (7/)216 spp. worldwide (Catalogue of Life), (1/)7 spp. in Brazil (CTFB/Chilopoda), (1/)3 in Mexico (Cupul-Magaña, BIOCYT, 2009).
MIMOPIDAE ‣ monotypic endemic to Shaanxi, China (Jiang et al., ZooKeys, 2020).
PLUTONIUMIDAE ‣ (2/)7 spp., in North America (Theatops posticus Say, 1821 up to N Mexico), S Europe and center China, Di et al., Zootaxa, 2010.
SCOLOPENDRIDAE ‣ (14/)481 spp. (Catalogue of Life) worldwide, (6/)34 in Brazil (CTFB/Chilopoda), (5)/21 in Mexico (Cupul-Magaña, BIOCYT, 2009).
SCOLOCRYPTOPIDAE ‣ (15/)120 spp. (Catalogue of Life), (4/)32 in Brazil (CTFB/Chilopoda), (4)/14 in Mexico (Cupul-Magaña, BIOCYT, 2009).
Despite being quite popular, the largest Chilopoda worldwide does not occurs in Brazil: Scolopendra gigantea L., 1758 is native from N Colombia, Venezuela, Trinidad, Isla Margarita, Curaçao and Aruba (Shelley & Kiser, Tropical Zoology, 2000). The largest Chilopoda in Brazil is S. viridicornis Newport, 1844, which occurs throughout South America (Souza & Chagas Jr., Dissertation, 2019), and is a very aggressive species, and has been recorded preying on bats (Noronha et al., Acta Amazonica, 2015).
ORDER SCUTIGEROMORPHA
(3:19/)56 spp. worldwide, very scarce in New World, with only 10 spp.
PSELLIODIDAE ‣ six spp. (Catalogue of Life) — however, based on Perez-Gelabert and Edgecombe (Novitates Caribaea, 2013), Würmli (Studies on Neotropical Fauna and Environment, 1978) and Stoev (Historia Naturalis Bulgarica, 2002), three are rejected here: Gonethella nesiotes Chamberlin, 1918, from the Cayman Islands, Gonethina grenadensis Chamberlin, 1918, from Grenada, and G. fijiana Chamberlin, 1920, from Fiji. The three remaining species are kept and recognized as valid here: Sphendononema chagualensis Kraus, 1957, known only the type collection in Marañon Valley in Peru; S. guildingii Newport, 1845, widely in New World; and S. rugosa Newport, 1844, restricted to Africa.
SCUTIGERINIDAE ‣ (1/)3 spp. in Scutigerina, known from South Africa, Madagascar, Swaziland, Lesotho and Zimbabwe (Giribet & Edgecombe, Invertebrate Systematics, 2003).
SCUTIGERIDAE ‣ (17/)50 spp. worldwide, with 8 native species in New World, being (3/)3 in Brazil: Dendrothereua linceci Wood, 1867 (S USA to Caribben and North Colombia, Edgecombe & Giribet, Cladistics, 2009 | Jiménez & Amazonas Chagas-Jr, CheckList, 2022), Theruella peruana Charberlin, 1955 (Peru), Edgethereua (2, Argentina and Chile), Scutigera sanguinea Meinert, 1886 (Argentina), S. parcespinosa Bücherl, 1949 (Brazil), Thereuoquima admirabilis Bücherl, 1949 (Brazil) and Brasiloscutigera viridis Bücherl, 1939 (Brazil), based on Porta & Giribet (Invertebrate Systematic, 2024).
29.4 DIPLOPODA CLASS ‣ (178:2,413/)14,026 spp. worldwide and (25:171/)584 spp. in Brazil, numbers based on a compilation of the data below analyzed by order. For Brazil, the data come primarily from the CTFB/Diplopoda, but are strongly complemented by additional studies that add further records and newly described species. The general reference Hoffman, Golovatch & Morais (Amazoniana, 1998), for Neotropical Diplopoda, is indicated in our text as HGM.
Golovatch & Kime (Soil Organisms, 2009) brings many observations about the group's ecological adaptations. Mexico has (39:117/)498 spp. (BOOK). A excelent work about Diplopoda in Neotropics found in Hoffman, Golovaych, Adis & Morais (Amazoniana, 1996). Distribution data for many genera can be consulted in Enghoff et al (Diplopoda - taxonomic overview, 2023), an outdated list of families can be consulted in Datacms, and an old checklist of millipedes from North America to Panama can be found in Hoffman (Checklist of the Millipeds of North and Middle America, 1999), used here in many instances of distributional information.
Many Diplopoda are invasive in Brazil, with emphasis on 14 species (Acta Amazonica, 2022 | ASEF, 2022): Ommatoiulus moreleti (Julidae, native to Iberian), Cylindroiulus britannicus (Julidae), Cylindroiulus truncorum (Julidae), Chondromorpha xanthotricha (Paradoxosomatidae, native to S Asia), Oxidus gracilis (Paradoxosomatidae, native to SE Asia), Orthomorpha coarctata (Paradoxosomatidae), Prosopodesmus jacobsoni (Haplodesmidae), Trachyjulus calvus (Cambalopsidae), Glyphiulus granulatus (Cambalopsidae), Trigoniulus corallinus (Pachybolidae), Leptogoniulus sorornus (Pachybolidae), Epitrigoniulus cruentatus (Pachybolidae), Paraspirobolus lucifugus (Spirobolellidae), and Rhinotus purpureus (Siphonotidae).
Some worldwide checklists includes Diplopda in Europe (1,619 spp., being 593 in Julida, 534 Chordeumatida, and 492 colectively in Polyxenida, Glomerida, Platydesmida, Siphonocryptida, Polyzoniida, Callipodida, Polydesmida, EJT | EJT | NHBS), subterranean Milipedes from Iberian Peninsula (Reboleira & Enghoff, Zootaxa, 2017), Diplopoda from Madagascar (266 spp., Wesener & Enghoff, The New Natural History of Madagascar, 2022), and Diplopoda from mainland China (Golovatch & Liu, ZooKeys, 2020).
Twelve orders of millipedes are hitherto reported from the African continent (Stoev & Akkari, ZooKeys, 2025): Polyxenida, Sphaerotheriida, Glomerida (unknwon in Afrotropics), Siphonophorida, Polyzoniida (only in South Africa and Madagascar), Polydesmida, Chordeumatida, Stemmiulida, Julida (unknwon in Afrotropics), Spirostreptida, Siphonocryptida (1, Hirudicryptus canariensis (Loksa, 1967) from the Canary and the Madeiran archipelagos), Spirobolida (), and Callipodida (1 sp., Morocco).









A. PENICILLATA
A single order worldwide.
ORDER PENICELLATA
(4:32/)184 spp. (Catalogue of Life) in Synxenidae (3/10, South Africa, Australia, New Guinea, South America, perhaps also the Mediterranean, HGM), Hypogexenidae (at least one sp., known in Brazil and Argentina, Grokipedia | Sousa et al., International Journal of Speleology, 2021), Lophoproctidae (7/46, Catalogue of Life, widely worldwide) and Polyxenidae (24/129, also widely worldwide, Catalogue of Life) widely distributed.
(8/)12 spp., in all four families, occur in Brazil (Ishii, Amazoniana, 1999): Synxenidae (1/1, Phryssonotus-1), Hypogexenidae (1/1, Grokipedia), Lophoproctidae (Ancistroxenus and Lophoturus one each, the former endemic to Brazil), and Polyxenidae (Macroxenus-1, Macroxenodes-3, Monographis-1, Polyxenus-3). Mexico has (2)/4 spp.
B. CHILOGNATHA/PENTAZONIA
Three orders worldwide.
ORDER GLOMERIDESMIDA
39 spp. in Termitodesmidae (1/5, tropical Asia), and Glomeridesmidae (2/31), from in Mexico (1), Guatemala, Panamá, Puerto Rico, Jamaica, Haiti, Lesser Antilles, parts of Asia, N Brazil (Glomeridesmus spelaeus Iniesta & Wesener, 2012, troglobic from ferriculous caves in SE Pará state, and at least 3 undescribed species, Brazil Cave Fauna, all accepted here), French Guiana and Ecuador. Termitodesmidae is the only myriapod family known to depend on insects in such a commensal relationship (Wikipedia). Glomeridesmoides (2) is endemic to French Guiana (Millibase).
ORDER GLOMERIDA
(3:36/)370 spp. (Catalogue of Life), all of these from southern Europe, Protoglomeridae up to Morocco and Glomeridae also in SE Asia and from California to Guatemala. Three genera occur in New World, Onomeris (3) and Trichomeris (1, Alabama) occur only in SE USA, and the single genus Glomeroides (16) is represented by
one species in Califomia and several in Mexico and Guatemala.
ORDER SPHAEROTHERIIDA
5 families and (25/)423 spp. (Catalogue of Life) from S Africa, Madagascar, S & SE Asia, Australia and New Zealand (Wikipedia).
C. CHILOGNATHA/HELMINTHOMORPHA/COLOBOGNATHA
Four order worldwide, one unknown in New World, one in New World but unknown in Brazil, and two in Brazil. (2:5/)11 spp. in Brazil, without report in CTFB.
ORDER SIPHONOCRYPTIDA
A single family, Siphonocryptidae, and (2/)7 spp., scattered in Canary Islands, Caucasus, China, peninsular Malaysia and Sumatra in Indonesia (Wikipedia).
ORDER PLATYDESMIDA
Two families and (16/)80 spp.: Andrognathidae from USA, Mexico (1/1), China, Japan, Italy, Greece and Portugal, and Platydesmidae from Mexico (1/10) to Panama.
ORDER SIPHONOPHORIDA
(2:18/)135 spp. (Catalogue of Life). Siphonorhinidae has five genera, three in Asia and South Africa, Illacme in California (with second leggest animal of world, with 618 legs, Wikipedia) and Notorhinus in Chile (Novataxa). Siphonophoridae has (12/)110 spp. widely in World, 4 genera in New World (Columbianum, Siphonacme, Siphonocybe, Siphonophora), with (3/)8 spp. in Brazil (Read H.J. & Enghoff H., EJT/2018, EJT/2019) and (2/)3 spp. in Mexico.
ORDER POLYZONIIDA
Three families and (28/)126 spp. Polizoniidae (4 genera in New World, 3 in W USA, 1 in E USA to SE Canada) and Hirudisomatidae (2 genera in Nw World, Mexiconium-1 in Mexico, Octoglena-4 in W & SE USA) occur in northern Hemisphere, and Siphonotidae raches in South America, Africa, SE Asia and New Zealand, unknown in North America, Mexico, America Central and Caribean. In Mexico there are a single Mexiconium. Siphonotids are represented in South America by the apparently native genus Burinia which occurs also in South Africa, and Siphonotus with two species endemic in Brazil. Rhinotus includes only introduced species in Brazil and the Caribbean (HGM). Brazil includes 3 spp., in Siphonotus-2 and Burinia-1 (Iniesta, L.F.M. et al., Pap. Avulsos Zool., 2021).
region
Eumillipes persephone Marek, 2021, in the latter family, is endemic to Western Australia, has 1,306 legs, the species with the most legs on Earth and the first millipede discovered to have 1,000 legs or more (Wikipedia).
D. CHILOGNATHA/HELMINTHOMORPHA/EUGNATHA/JULIFORMEA
Three orders, two in Brazil.
ORDER JULIDA
(23:252/)1,480 spp. worldwide (Catalogue of Life), 14 families only in Old World, five endemic to USA: Aprosphylosomatidae (1/1, Oregon), Chelojulidae (1/1, Idaho), Paeromopodidae (2/16, Oregon to California), Telsonemasomatidae (1/1, Oregon) and Zosteractinidae (2/2, C & E USA); three from USA/Canada and other areas in Old World: Nemasomatidae (two genera in New World, both in E North America and disjunct along Old World), Okenabatidae (2 spp., Japan and E USA one each) and Blaniulidae (only Virgoiulus in New World, SE USA); and Parajulidae — a family of apparently specialized julidans is most diverse and abundant in North America with a explosive radiation (at least 28 genera), a few genera occur also in East Asia and southward through Middle America
as far as Guatemala, where the single genus Thriniulus has been found (HGM). (7/)17 spp. in Mexico, including three endemic genera.
■ endemic families in New World: Aprosphylosomatidae (1/1, USA), Chelojulidae (1/1, USA), Paeromopodidae (2/16, USA), Telsonemasomatidae (1/1, USA) and Zosteractinidae (2/2, USA).
ORDER SPIROBOLIDA
(13:152/)1,230 spp. worldwide, nine families in New World: six outside South America — Spirobolidae from North America and Asia (5/24 spp. in New World, from SE Canada to Guatemala, includes Floridobolus), Atopetolidae (explosive radiation in USA and Mexico-8/19); Typhlobollelidae (4/5, endemic to Mexico), Hoffmanobolidae (1/1, Oaxaca), Allopocockiidae (5/7, Mexico to El Salvador), Messicobolidae (2/27, Mexico to Honduras) — and remaining three in to continent: Pachybolidae (Africa, Madagascar, India, Sri Lanka to Borneo, and Brazil), Rhinocricidae (Indo-Australian area, westem Nearctic, and the Neotropics, HGM, 6/69 in Brazil by CTFB/Diplopoda, unknown un USA and Canada) and Spirobolellidae (1/2 in Brazil by CTFB/Diplopoda | HGM, unknown out north of Panama).
Except for two Brazilian genera of uncertain status (Epitrigoniulus-1 and Neptunobolus-2), Pachybolidae are introductions from SE Asia, and occur most commonly in gardens and plantations, often in great numbers (HGM). Within these figures, Brazil has (3:9/)74 spp., and Mexico has (9:25/)81.
Bioluminescent Diplopoda belongs 10 spp., Paraspirobolus lucifugus Gervais, 1836 (Spirobolellidae) from Japan, and nine in Polydesmida from W North America.
■ endemic families in New World: Hoffmanobolidae (1/1, Mexico) and Typhlobolellidae (5/6, Mexico).
ORDER SPIROSTREPTIDA
The second largest order of Diplopoda, this dominantly tropical assemblage includes the largest known millipedes as well as some of the smallest (HGM). (12:268/)2,152 spp. worldwide (Catalogue of Life), only four in New World: Choctellidae endemic to USA (1/2, Tennessee to Alabama); Cambalidae for USA (14 genera, 11 endemic to California, 10 monotypic, two in Caifornia up to Oregon and Washington, and Cambala from E USA to Mexico), Mexico (2/4 in endemic Mexicambala and Cambala) and Belize (1/1 in Jarmika, endemic), Hawaii, New Zealand, Australia and Chile (2, in Zinagon); and two in Brazil, Pseudonannolenidae (2/26 in Brazil, CTFB/Diplopoda, unknown north of Costa Rica, Camballoma endemic to Haiti) and Spirostreptidae (21/69, CTFB/Diplopoda | Urostreptus atrobrunneus, only Orthoporus in Mexico or USA), both widely in New World, Africa to Arabia. For notes of biogeography of Pseudonannolenidae, see Iniesta & Ferreira (Zootaxa, 2014). Mexico includes 37 spp. in Spirostreptidae and 4 in Cabalidae.
■ endemic families in New World: Choctellidae (1/2, USA).
E. CHILOGNATHA/HELMINTHOMORPHA/EUGNATHA/NEMATOPHORA
Three orders worldwide.
ORDER CALLIPODIDA
(10:43/)163 spp. worldwide (Catalogue of Life), two in New World: Abacionidae (E Asia, Tetracion and Delophon endemic to USA, Abacion in E USA and N Mexico) and Schizopetalidae (9 genera, all only in USA except Colactis up to N Mexico, and Colactoides-1 endemic to Mexico), both from USA to N Mexico (5/7 spp. in country).
ORDER CHORDEUMATIDA
(59:344/)1,470 spp. (Catalogue of Life), with 14 families in New World in a explosive radiation in W USA (with six endemic families and three near endemic): Caseyidae (12/57, W USA, E Russia-1/1), Striariidae (16/52, SW Canada to California), Tingupidae (2/11, USA, SW Canada, Alaska, Zootaxa), Rhiscosomididae (1/7, Alaska to California, including SW Canada), Microlympiidae (1/1, Washington, Zootaxa), Urochordeumatidae (1/1, Washington), Adritylidae (1/3, W USA), Apterouridae (1/2, California), Buotidae (1/1, E USA), Branneriidae (1/2, SE USA), Trichopetalidae (4/25, 3 genera in USA, one up to SE Canada, and Mexiterpes-7 endemic to Mexico), Conotylidae (11/51, USA, 3 genera up to Canada, Austrotyla up to Mexico), Cleidogonidae (8/135, NE USA to Panama, Pseudotrema-37 endemic to E USA), and Eudigonidae in Argentina and Chile in South America. (4/)52 spp. in Mexico.
■ endemic families in New World: Adritylidae (1/3, USA), Apterouridae (1/2, USA), Branneriidae (1/2, USA), Buotidae (1/1, USA), Microlympiidae (1/1, USA) and Urochordeumatidae (1/1, USA).
ORDER STEMMIULIDA
A single family, Stemmiulidae, with (7/)159 spp. (Catalogue of Life), from New Guinea, Indonesia, SE Asia, central Africa and tropical America. In Brazil has only 4 spp. in Stemmiulus (all endemic, 3 in Amazonas and 1 in Pará state, Iniesta & Ferreira, Zootaxa, 2015). Mexico has (1/)3 spp., all in Prostemmiulus. Unknown in USA.
F. CHILOGNATHA/HELMINTHOMORPHA/EUGNATHA/MEROCHAETA
A single order.
ORDER POLYDESMIDA
(37:1,186/)6,005 spp. worldwide (Catalogue of Life, excludes Brazilian additions) and (12:124/)384 in Brazil. In New World there are 21 families, 9 unknown in Brazil: Polydesmidae (S Canada to SW USA, Japan and India to Papua New Guinea, 6/32 in New World, unknown natively in Mexico), Xystodesmidae (S Alaska to El Salvador and Asia, a explosive radiation in E USA along at least 47 endemic genera, remarkable of these by Eurymerodesmus-35 and Brachoria-26, 2/68 in Mexico, in Rhysodesmus and Stenodesmus), Nearctodesmidae (5/8, N British Columbia to SW USA, Ergodesmus in Illinois, Sakophallus in WC Mexico), Holistophallidae (7/11, C Mexico to Honduras and El Salvador), Sphaeriodesmidae (12/90, S USA to Panama, Caribbean), Tridontomidae (2/4, Guatemala, including the only species in the infraclass Helminthomorpha without gonopods, Wikipedia | BHL), Rhachidesmidae (16/56, Mexico to Costa Rica), Macrosternodesmidae (Europe, Chaetaspis-5 in SE USA), and Dorsoporidae (Panama), all absent in South America.
In South America are 12 families, all in Brazil: Aphelidesmidae (4/12, CTFB/Diplopoda, unknown north of Mexico), Chelodesmidae (a explosive radiation in Neotropics, 62/225 in Brazil: CTFB/Diplopoda | six Eucampesmella | Hoffmanopeltis gen. nov. | 3 Santalodesmus | Tessarithys exacuminatus, Iguazus robustus | Cayenniola albaserrata | 3 Leptherpum | Strongylosomides troglobius, Rotundotergum elevatum | 2 Tupadesmus | Pandirodesmus zogbiae, unknown in USA), Cryptodesmidae (10/28, CTFB/Diplopoda, unknown north of Mexico), Cyrtodesmidae (1/1, CTFB/Diplopoda, unknown north of Costa Rica), Dalodesmidae (1/1, CTFB/Diplopoda), Dobrodesmidae (1/1, CTFB/Diplopoda | Shear et al., Zootaxa, 2016 — known only from Ituaçu municipality, southern Bahia state), Haplodesmidae (1/2, CTFB/Diplopoda, unknown north of Panama), Oniscodesmidae (1/8, CTFB/Diplopoda, known north of Panama only by Ligiodesmus in Mexico), Paradoxosomatidae (17/56, CTFB/Diplopoda | 3 Catharosoma | Iulidesmus silvestrii, Onciurosoma troglobium, unknown north of Costa Rica), Platyrhacidae (1/1, CTFB/Diplopoda, worldwide from Nicaragua to South America and Old World, Zootaxa), Pygrodesmidae (15/25: CTFB/Diplopoda | Pseudoporatia kananciue, known in USA only by Psochodesmus in Florida), and Trichopolydesmidae (10/24, subcosmopolitan: CTFB/Diplopoda | Phaneromerium troglopterygotum, Moojenodesmus schubarti | Adisia pifae, Moojenodesmus arenicola). (59/)262 spp. in Mexico.
Trichopolydesmidae is mainly in the Northern Hemisphere, both temperate and, especially, tropical. They are only marginally represented in Java, Indonesia and Wallacea in the southeast, being totally absent from New Zealand, Australia, the southern half of Africa, and Madagascar, also absent from the Nearctic. The bulk of trichopolydesmid diversity, both generic and species, is taken up by what has until recently been considered as an independent pantropical family Fuhrmannodesmidae (Golovatch et al., ZooKeys, 2014).
Bioluminescent Diplopoda belongs 10 spp., nine Motyxia in Xystodesmidae from W USA (Shimomura & Yampolsky, BOOK, 2019), at Polydesmida, and one Spirobolellidae (Spirobolida) from Japan.
■ endemic families in New World: Dobrodesmidae (1/1, Brazil), Dorsoporidae (1/1, Panama) and Tridontomidae (2/4, Guatemala).
G. UNPLACED ORDER
Three species worldwide.
ORDER SIPHONIULIDA
Order with only three species in Siphoniulidae: Siphoniulus alba Pocock, 1894, from E Sumatra island in Indonesia (known only two fragments), and two from S Mexico and Guatemala (Sierwald, Zool. Syst. Evol. Research, 2001 | PeerJ).
29.5 PAUROPODA CLASS ‣ (12:56/)1,021 spp. worldwide (Catalogue of Life — used for all the global data below) in two orders. (4:11/)63 spp. in Brazil (CTFB/Pauropoda).
ORDER HEXAMEROCERATA
A single family, Millotauropodidae, with 8 spp. in a single genus, Millotauropus (Minelli, BOOK, 2011), from Brazil (M. acostae Scheller, 1997 — unique record in New World), Africa to Madagascar, Seychelles and Japan.
ORDER TETRAMEROCERATA
(11:55/)1,013 spp. worldwide. 7 families occur in New World, and four are not recorded from: Antichtopauropodidae (1/1, W Australia), Sphaeropauropodidae (1/14, Nepal to Borneo and Japan), Eirmopauropodidae (1/1, New Zealand) and Collinapauropodidae (1/3, Japan, Reunion and Philippines). (3:10/)62 spp. in Brazil.
AMPHIPAUROPODIDAE ‣ only Amphipauropus (2), in from Canada in New World, Iceland, Norway, Sweden, Denmark, Germany, France and Japan.
BRACHYPAUROPODIDAE ‣ (6/)33 spp.: Aletopauropus (2, USA, Japan), Borneopauropus (5, Borneo, Australia, New Zealand), Brachypauropoides (7, Madagascar, New Zealand), Brachypauropus (9, USA, Germany, Poland, France, Austria, Switzerland, Romania, Spain, Italy, Greece), Deltopauropus (4, USA, Japan) and Zygopauropus (1, USA).
DIPLOPAUROPODIDAE ‣ (2/)4 spp., in Diplopauropus (3, USA, Virgin Island one ach) and Adelphopauropus (1, endemic to Manaus region in N Brazil — Scheller, Zootaxa, 2013).
EURYPAUROPODIDAE ‣ (4/)75 spp.: Acopauropus (12, Europe, N Africa, Koreas), Eurypauropus (5, USA and Japan), Samarangopus (35, Africa, Asia and Oceania) and Trachypauropus (10, Europe, Israel).
HANSENAUROPODIDAE ‣ (3/)7 spp.: Antillauropus (1, Jamaica), Hansenauropus (3, Panama, Madagascar, New Zealand) and Virginopauropus (2, Virgin Islands, Sierra Leone).
PAUROPODIDAE ‣ (30/)836 spp. in both Hemispheres. (7/)48 spp. in Brazil.
At least 14 genera in New World (Brazilian in bold, 7/48): Allopauropus (96, subcosmopolitan, 11 in Brazil), Cauvetauropus (5, Brazil/1, France, Sri Lanka, Maghreb, Ivory Coast, Madagascar), Decapauropus (377, subcosmopolitan, 27 in Brazil), Desmopauropus (1, USA), Donzelotauropus (27, northern Hemisphere), Hemipauropus (20, Jamaica, Colombia, Brazil/2, Caribbean, Italy, Greece, Sri Lanka, New Caledonia, Guam, Madagascar, Mauritius, mainland Africa), Juxtapauropus (9, Israel, Morocco, Jamaica, Argentina, Chile and New Zealand, reported in Brazil in Minelli/2011, however not listed in CTFB), Pauropus (52, subcosmopolitan), Perisopauropus (4, Africa, Chile and 1 in Brazil), Propepauropus (1, USA), Rabaudauropus (5, Chile, Sri Lanka, Malaysia, New Caledonia, Mauritius, Seychelles, Japan, Marocco, France, Romania, Greece), Scleropauropus (15, Brazil/5, Mexico, USA, Europe, Madagascar, mainly Africa), Stylopauropoides (25, Brazil/1, Argentina, Crozet, Ivory Coast, Madagascar, Australia, New Zealand, New Caledonia) and Stylopauropus (23, Europe and northern Africa to SE Asia, Canada and USA).
At least 8 genera only in Old World: Afropauropus (1, Guinea), Eburniopauropus (2, Ivory Coast), Ferepauropus (2, disjunct Sierra Leone and New Caledonia), Hystrichopauropus (1, France), Kionopauropus (3, SE Asia), Multipauropus (1, Greece), Nesopaurpus (8, Gabon, Seychelles, Sri Lanka, New Zealand) and Pounamopauropus (1, New Zealand).
POLYPAUROPODIDAE ‣ (3/)30 spp.: Fagepauropus (2, Canada, Morocco, Mongolia, Japan, Gambia), Polypauropoides (12, USA, Brazil/6, Argentina, France, Ivory Coast, Mauritius, Sri Lanka) and Polypauropus (15, Brazil/6, Argentina, USA, Europe, Africa, Madagascar, Mauritius, Reunion, Sri Lanka, Australia). (2/)12 spp. in Brazil.
29.6 SYMPHYLA CLASS ‣ no order, two families and 243 spp. worldwide (Catalogue of Life), (2:4/)12 in Brazil (CTFB/Symphylla | Scheller, Amazoniana, 2007). Mexico has (5/)15 spp. (GBIF). South America includes 5 genera: Scolopendrellopsis (4) in Brazil-2, Argentina-2 and Paraguay-1; Symphyllela (6) in Argentina-3, Brazil-2, Venezuela-2 and Paraguay-1; Ribautiella (2) in Brazil (also in Africa); Scutigerella (1) in Argentina and Chile; and Hanseniella (8) in Brazil-6, Argentina-5 (one recent, ZE/2024), Venezuela-2, Uruguay-1, Paraguay-1, Ecuador-1, Chile-1 and Colombia-1 (Scheller, Amazoniana, 1992).
SCOLOPENDRELLIDAE ‣ (9/)102 spp., composed in New World by Geophilella (2, Europe and North America), Ribautiella (10, 8 in Africa and two in Brazil), Scolopendrellopsis (17, subcosmopolitan, Brazil and Mexico two each) and Symphyllela (44, subcosmopolitan, two in Brazil, 3 in Mexico).
Hanseniella guerreroi Porta, Parra-Gómez & Fernández, 2024 from Isla Grande de Tierra del Fuego and Isla de los Estados in Argentina represents the southernmost record for a Symphyla worldwide (Osvaldo Porta, A. et al., Zoosystematics and Evolution, 2024).
SCUTIGERELLIDAE ‣ (5/)141 spp., three genera in the New World: Hanseniella (c. 80, subcosmopolitan, 5 in Brazil, 3 in Mexico), Scopoliella (1, endemic to Mexico, possibly in Colombia, SEE) and Scutigerella (35, subcosmopolitan but not recorded in Brazil, 1 in South America, six in Mexico).
29.7 OLIGOSTRACA CLASS ‣ a complex class comprising 11 orders, including endo and ectoparasitic forms, as well as free-living species, mainly bivalved aquatic arthropods found in all types of aquatic ecosystems. (51:796/)5,823 spp. worldwide (25:190/)453 in Brazil. In Mexican seas, 883 spp. of Ostracoda have been reported — 506 in the Gulf of Mexico and Caribbean, and 418 in the Pacific Ocean — although approximately 45% may be synonyms (Bousquets et al., Conabio, 2000).
Some sources, such as Wikipedia, consider Oligostraca to be a superclass comprising three distinct classes (SEE). Here, however, we treat it as a single class, with the classes recognized by Wikipedia regarded as subclasses, alongside other already established subclasses.




SUBCLASS BRANCHIURA
A single order, Arguloida, (4/)168 spp. in a single family, Argulidae (Catalogue of Life), with four genera: Argulus, Chonopeltis, Dipteropeltis and Dolops (Binoculos excluded here), primarily ectoparasites of freshwater fish (c. 3/4 of diversity), but in some exceptional cases specimens have been found attached to marine fishes, and more rarely still, to amphibians and reptiles. Neotropical region has one of the highest diversity of species (31 in South America) and, with the exception of A. peruvianus Oliva, Duran & Verano, 1980 (endemic to coast of Peru near Callao), all the remaining species occur in freshwater. In Brazil occur (3/)23 spp. (Aguiar et al., Zootaxa, 2017, Chonopeltis is found only in Africa), with 6 endemic. (1/)6 spp. in Mexico (Bousquets et al., Conabio, 2000).
SUBCLASS PENTASTOMIDA
(9:25/)131 spp. worldwide (Catalogue of Life | Christoffersen and De Assis, Zoologische Mededelingen, 2013). Brazil has the largest diversity of this group in New World, with (7:11/)17 spp. (CTFB/Pentastomida).
ORDER CEPHALOBAENIDA
Two spp., one valid (Cephalobaena recurvocaudata Motta, 1963, from snakes of South America) and other doubtful (Bothropsiella bicornuta Cavalieri, 1967, known only a female collected in a snake form Argentina), both in the single family Cephalobaenidae.
ORDER RAILLIETIELLIDA
(2/)42 spp. in a single family, Raillietiellidae. Raillitiella has 12 spp. in New World, 4 in Brazil (none endemic). Brazilian not recorded in species are from North America (2, one also in Madagascar) plus Puerto Rico, Mexico and Colombia one endemic each. One genus endemic to Australia.
ORDER REIGHARDIIDA
(2/)3 spp. in a single family, Reighhardiidae. Only a single species in New World, the cosmopolitan Reighardia sternae (Diesing, 1863), collected mainly in sea birds, in Brazil in sacs of Larus dominicus.
ORDER POROCEPHALIDA
(19/)84 spp. in 4 families. All 4 families (Linguatulidae, Porocephalidae, Sebekidae, and Subtriquetridae), and all eight genera in New World occur in Brazil (15, 3 endemic). Brazilian not recorded in species are in Caribbean (3), Mexico (2), USA (1), and one in Crocodylus acutus range, from northern South America to Florida, America Central and Caribbean. Sambonia, cited in work for Uruguay, is now Porocephalus crotali (Carbajal-Márquez, Journal of Parasitic Diseases, 2018).
SUBCLASS MYODOCOPA (OSTRACODS)
(5:175/)1,173 spp. worldwide (according to the data below) and (4:7/)9 in Brazil. Traditionally, Myodocopa and Podocopa have been classified as subclasses within the class Ostracoda, although there is some question about how closely related the two groups actually are. The Myodocopa are defined by possession of a poorly calcified carapace, and 8–9 articles in the exopod of the second antenna.[2] The ventral margin of the carapace is not concave, and the valves do not overlap to a great extent (Wikipedia).
ORDER MYODOCOPIDA
(2:94/)738 spp. worldwide (Catalogue of Life). Many species of the Myodocopida have lateral compound eyes. (2/)2 spp. in Brazil, both in Cylindroleberididae (CTFB/Myodocopida).
ORDER HALOCYPRIDA
(3:81/)435 spp. worldwide (Halocyprididae, Polycopidae and Thaumatocyprididae, Catalogue of Life), primarily planktonic, all families in Brazil, with (5/)7 spp. in country (CTFB/Halocyprida).
SUBCLASS MYSTACOCARIDA
Tiny worm-like crustaceans, in a single order and (2/)13 spp. in a single family Derocheilocarididae (Olesen, Guide to the Identification of Marine Meiofauna, 2020): Ctenocheilocaris (5, 1 in coastline of Chile, 3 in Brazil, and 1 in W Australia) and Derocheilocaris (8, E North America, SW coast of Europe to Africa and Israel).
SUBCLASS PODOCOPA (OSTRACODA)
Three order with (34:590/)4,338 spp. worldwide, (34:168/)401 in Brazil.
ORDER PLATYCOPIDA
A single family, Cytherellidae, with (5/)117 spp. (Catalogue of Life). (3/)8 spp. in Brazil (CTFB/Platycopida).
ORDER PALAEOCOPIDA
A single living family, (2/)3 spp., endemic to high-energy shallow marine environments of New Zealand (Wikipedia).
ORDER PODOCOPIDA
(32:583/)4,218 spp., the only one with freshwater species, with (CTFB/Catalogue of Life). CTFB lists 34 families in Brazil, but only 12 are accepted based on the Catalogue of Life (Bairdiidae, Bythocyprididae, Bythocytheridae, Candonidae, Cyprididae, Cytherettidae, Darwinulidae, Ilyocyprididae, Krithidae, Macrocyprididae, Paradoxostomatidae, and Thaerocytheridae) — thus, we count (12:165/)393 spp. in Brazil (CTFB/Podocopida). (295/)2,420 spp. of former Ostracods are freshwaters worldwide, within 20 families. 451 spp. are known in Neotropical freshwaters, domined by Cyprididae (Meisch, C. et al., EJT, 2024).
29.8 THECOSTRACA CLASS ‣ although widely accepted as separate groups, here we treat Tantulocarida as a branch of Thecostraca and provide a unified discussion for both (Bernot, J.P. et al., Molecular Biology and Evolution, 2023). (60:331/)2,214 spp. worldwide and (17:47/)73 in Brazil (in Cirripedia and Tantulocarida), numbers based on a compilation of the data below analyzed by order, in 4 high lineages. 55 spp. of Thecostraca occur in Mexico (none Tantulocarida), 48 in Pacific coast and 7 in Atlantic Coast (Bousquets et al., Conabio, 2000).
Facetotecta clade contains a single genus, Hansenocaris, known only from the tiny planktonic nauplii y-larvae. These larvae have no known adult form, though it is suspected that they are parasites, and their affinity is uncertain. Some researchers believe that they may be larval tantulocaridans. No larval tantulocaridans are currently known (Wikipedia/Thecostraca).




SUBCLASS FACETOTECNA (planktonic)
A single family, Hansenocarididae, with 17 spp. in Hansenocaris (Catalogue of Life), unknown in Brazil.
SUBCLASS ASCOTHORACIDA (parasites, WIKI)
As ascothoracicans no longer require a typical crustacean body plan. After becoming internal or semi-internal parasites, they lost locomotory appendages, abandoned evident body symmetry, and greatly reduced or completely eliminated sensory and muscular structures. As a result, the body is essentially transformed into an attachment organ combined with a highly specialized reproductive system. (6:25/)127 worldwide, unknown in Brazil.
ORDER DENDROGASTRIDA
(3:9/)59 spp. worldwide (Catalogue of Life), parasitics in found throughout the world on Echinoderms, unknown in Brazil.
ORDER LAURIDA
(3:16/)68 spp. worldwide (Catalogue of Life), parasitics in found throughout the world mainly on Cnidarians and sometimes in Echinoderms, unknown in Brazil.
SUBCLASS CIRRIPEDIA (sessile)
Nine lineages. Barnacle adults are sessile; most are suspension feeders with hard calcareous shells, but the Rhizocephala are specialized parasites of other crustaceans, with reduced bodies. Barnacles have existed since at least the mid-Carboniferous, some 325 M years ago. (48:282/)2,031 spp. worldwide and (16:46/)72 in Brazil, numbers based on a compilation of the data below analyzed by order.
ACROTHORACICA/ORDER CRYPTOPHIALIDA
(1:2/)22 spp. (Catalogue of Life), burrowing into calcareous rocks and structures, such as limestone, shells, or corals, unknown in Brazil.
ACROTHORACICA/ORDER LITHOGLYPTIDA
(3:11/)52 spp. worldwide (Catalogue of Life), unknown in Brazil.
RHIZOCEPHALA (INFRACLASS, ORDER EQUIVALENT LEVEL)
(13:45/)300 spp. worldwide (Catalogue of Life), (2:5/)6 spp. in Brazil (CTFB/Crustacea). The family Lernaeodiscidae cited in the CTFB is synonymized with Peltogastridae according to B.K.K. Chan et al. (2021).
PHOSPHATOTHORACIA/ORDER IBLOMORPHA
(2:4/)8 living spp. worldwide (Catalogue of Life), barnacles that use calcium phosphate in their shell, and species that protect themselves against predators with poison, known from coasts of SE India, S Japan, Sumatra, Timor, Australia, New Zealand, Fiji and Samoa (MAP/Copepedia) — unknown in Brazil.
THORACICALCAREA/ORDER BALANOMORPHA
(14:127/)768 spp. worldwide (Catalogue of Life). (8:21/)40 spp. in Brazil (CTFB), in Archaeobalanidae (4/4), Balanidae (4/14), Chelonibiidae (1/3), Chthamalidae (2/3), Coronulidae (2/3), Platylepadidae (2/4), Pyrgomatidae (2/4), Tetraclitidae (4/5).
THORACICALCAREA/ORDER CALANTICOMORPHA
A single living family, Calanticidae, with (10/)71 spp. (Catalogue of Life), unknown in Brazil.
THORACICALCAREA/ORDER POLLICIPOMORPHA
(2:5/)79 spp. (Catalogue of Life), only one in Brazil (CTFB), in Lithotryidae.
THORACICALCAREA/ORDER SCALPELOMORPHA
(10:67/)626 spp. worldwide (Catalogue of Life), (4:16/)20 spp. in Brazil (CTFB), in Scalpellidae, Heteralepadidae, Lepadidae, and Poecilasmatidae. The family Oxynaspididae cited in the CTFB is synonymized with Heteralepadidae by Catalogue of Life.
THORACICALCAREA/ORDER VERRUCOMORPHA
A single family worldwide with (2:13/)105 living spp., (1:3/)5 in Brazil (CTFB).
SUBCLASS TANTULOCARIDA
(5:23/)39 spp., unplaced in a order. Tantulocaridans as a whole have been reported with a worldwide distribution but only four species collected in all New World waters (A. S. Petrunina & R. Huys (Journal of Crustacean Biology, 2020, a list of all species worldwide): Tantulacus coroniporus Arbizu & Petrunina 2018 and Aphotocentor kolbasovi Petrunina & Huys, 2020 (Cumoniscidae) in Atlantic Ocean (specifically from the Argentinian abyssal plain and the Campos Basin, off SE Brazil, respectively), Basipodella harpacticola Becker, 1975 and Rimitantulus hirsutus Huys & Conroy-Dalton, 1997 in Pacific Ocean (Peru-Chile trench, and Galapagos islands, Ecuador). Six species also occur in Drake passage in southern South America.
This sources includes the citation of the world's smallest arthropod, Serratotantulus chertoprudae Savchenko & Kolbasov, 2009, with a total body length of only 76 micrometres, or ~1/14 of a milimeter, from Indian Ocean.
29.9 MALACOSTRACA CLASS ‣ (719:7,367/)46,650 spp. worldwide (Catalogue of Life, data sectioned) and (290:917/)2,240 spp. in Brazil (CTFB/Malacostraca).
18 orders, mainly shrimp-like organisms, placed in three well defined groups: basal Leptostraca, Stomatocarida (Stomatopoda, Bathynellacea, Anaspidacea, Euphausiacea and Decapoda) and Peracarida (Amphipoda, Stygiomysida, Mysida, Tanaidaceae, Cuamcea and Isopoda), however six clades are unplaced within this groups: Thermosbaenacea, Mictacea, Bochusacea, Lophogastrida, Ingolfiellida and Spelaeogriphacea (Bernot, JP et al., Molecular Biology and Evolution, 2023). Decapoda, Amphipoda and Isopoda represents the bulk of Malacostraca. Brazil has the second largest number of orders worldwide, 14, being exceptions Anaspidacea, Stygiomysida, Thermosbaenaceae and Mictacea, after Australia.
Bathynellacea, Leptostraca, and Lophogastrida have no data in the CTFB. Mictacea, Ingolfiellida, and Spelaeogriphacea have no data in the Catalogue of Life.
Some checklist worldwide includes Decapoda from Madeira island (215 spp., Araújo & Wirtz, Spixiana, 2015), marine Isopoda from Iberian peninsula (39:220 spp., Junoy & Castelló, Boletin de Instituto Espanol de Oceanografia, 2003), marine Amphipoda from India (56:133/266 spp., Thacker, D et al., Zootaxa, 2023), Cladocera from S Mexico, Belize and N Guatemala (56 spp., Elías-Gutiérrez, M et al., Zootaxa, 2006), Cladocera from Cuba (70 spp., Elías-Gutiérrez & Varela, Crustaceana, 2009).
ORDER LEPTOSTRACA
(3:11/)70 spp. worldwide (Catalogue of Life). Some Nebalia occur in Brazil (Wakabara, Crustaceana, 1965). Only two species occur in Mexico, both restricted for to Gulf of Mexico (Bousquets et al., Conabio, 2000).
ORDER STOMATOPODA
(22:129/)515 spp. worldwide (Catalogue of Life). In Brazil occur (9:20/)47 spp. (CTFB/Stomatopoda), all marine.
ORDER BATHYNELLACEA
(81/)322 spp. in two freshwater families, numbers based on a compilation of the data below analyzed by family. (2:9/)10 spp. in Brazil, unknown in Mexico (Camacho, Zootaxa, 2006).
BATHYNELLIDAE ‣ (36/)112 spp. worldwide (Perina, G. et al, ZJLS, 2024), 5 genera in New World (Lopreto & Morrone, Zoologica Scripta, 1998): Austrobathynella (subantarctic areas, only a single species in Argentina at region), Bathynella (cosmopolitan, only Chile in region, with 2 spp.), Montanabathynella (3 N USA), Nannobathynella (2, one in SE Brazil, another in Africa) and Pacifibathynella (W North America and E Asia). Only one sp. in Brazil. Bathynellidae in North America includes only 7 known species: Bathynella (3, 1 in Colorado and 2 in California) and Pacificabathynella (4, California/1 and Montana/3) and Montanabathynella (3, Montana), by Camacho, Newell & Reid (Journal of Natural History 1, 2009 | Journal of Natural History 1, 2009).
PARABATHYNELLIDAE ‣ (45/)210 spp. worldwide (Camacho, Zootaxa, 2006 | Camacho et al., Journal of Natural History, 2020), 14 genera in New World, 11 in South America (Lopreto & Morrone, Zoologica Scripta, 1998) plus Califobathynella, Californibathynella and Texanobathynella in North America. (11/)19 spp. in South America, in Atopobathynella (4, Chile-1 and Oceania-3), Brasilibathynella (1, endemic to Brazil), Chilibathynella (3, California-1 ,Chile-1 and Australia-1), Ctenobathynella (6, Brazil-1, Africa-4, Israel-1), Hexabathynella (11, Brazil-1, Argentina-1, Madagascar-1, New Zealand-1, Europe-4, Australia-2, USA-1), Leptobathynella (2, Brazil-2, Argentina-1 and Paraguay-1), Noodtibathynella (2, Brazil-1 and Argentina-1), Odontobathynella (1, endemic to Brazil), Parvulobathynella (3, Brazil-1, Chile-1 and Paraguay-1), Psallidobathynella (1, Venezuela) and Thermobathynella (2, Brazil-1, Africa-1). (8/)9 spp. in Brazil.
ORDER ANASPIDACEA
(12/)25 spp. in five families: Anaspididae (3/12), Koonungidae (2/2) and Psammaspididae (2/2) are found in Australia and Tasmania; Patagonspididae (1/1, aquifers in Rio Negro, SEE) is restricted to Argentina; and Stygocarididae (4/8) occur in Argentina (3, Parastygocaris/SEE), Chile (1 Oncostygocaris/SEE, 1 Stygocaris/SEE), Australia (1 Stygocaris, SEE) and New Zealand (1 Stygocarella/SEE, 1 Stygocaris/SEE). All the Neotropical species (2:4/6, Plazi) are stygobionts that inhabit aquifers and hyporheic interstitial environments, and mostly are cited only for their type locality (Plazi).
■ endemic families in New World: Patagonaspididae (1/1, Argentina).
ORDER EUPHAUSIACEA
(2:13/)88 spp. in two families worldwide (Catalogue of Life), full marine: Euphausiidae (10/87) and Bentheuphausiidae (Bentheuphausia amblyops G.O. Sars, 1885, a bathypelagic krill living in deep waters below 1,000 m), both in Brazil with (6/)30 spp. in country (Gibbons et al., South Atlantic Zooplankton, 1999 | CTFB | Demoner, Thesis, 2017). For some notes for possibly new species in Atlantic Ocean, see Kulagin, D.N. et al. (Invertebrate Systematics, 2023).
7 genera do not occur in Brazil: Bentheuphausia, Hansarsia, Meganyctiphanes (1, North Carolina/Mauritania line nortwards in Northern Atlantic, EWO), Nocticula, Nyctiphanes (Pacific coast of North and South America, SW Africa Atlantic, northern Europe, southern Australia, MAP), Pseudeuphausia (2, Somalia to Australia in Indic Ocean, Japan to New Caledonia in Pacific Ocean, EWO) and Tessarabrachion (1, Japan to California in northern Pacific, EWO).
ORDER DECAPODA
(272:2,805)18,319 spp. worldwide (Catalogue of Life), numbers based on a compilation of the data below analyzed by order, from caves to abyssal zones in sea. 12 lineages in two high clades: Dendrobranchiata and Pleocyemata. Three patterns of body was found in this order: shrimp-like (Dendrobrachiata, Stenopodidea, Carines, Procarididea, Glypheidea), crab-like (Anomura and Brachyura) and lobster-like (Gebiidea, Axiidae, Achelata, Polychelida and Astacida).
(114:423/)1,075 spp. in Brazil, in all Decapoda lineages except Procaridoidea and Glypheidea, numbers based on a compilation of the data below analyzed by order. Mexico includes (114:536/)1,776 spp. of Decapoda, 1,597 marine and 177 freshwater (Álvarez et al., Revista Mexicana de Biodiversidad, 2014: HM | Kawai & Cumberlidge, BOOK, 2014: KC). A total of (26/)174 spp. occur in freshwater environments in Brazil, in in Acetes (A. paraguayensis Hansen, 1919), (7/)41 in Caridea, (1/)14 in Astacidae, (1/)68 in Aegla, and (16/)50 in Brachyura.
For freshwater decapods in South America, see Kawai (A Global Overview of the Conservation of Freshwater Decapod Crustaceans, pg. 10, 2016). For deep-sea Decapods in Brazil (below 500m), see Cardoso et al. (Catalogue of Typical Deep-Sea Decapod Fauna from Brazilian Waters, 2021).
DENDROBRACHIATA
(10:84/)593 spp. worldwide (Catalogue of Life), sister of remaining Decapoda (Pleocyemata), all marine except Acetes paraguayensis Hansen, 1919 (Sergestidae) from Venezuela and Ecuador to E Brazil and NE Argentina, only Dendrobranchiata known in the world that inhabits totally fresh water (Sergestidae, Planeta Invertebrados). (8:39/)73 spp. in Brazil, in Acetidae (1/4, largest diversity worldwide, Vereshchaka, AL et al, 2016, MAP), Aristeidae (6/8), Benthesicymidae (5/8), Penaeidae (10/24), Sicyoniidae (1/7), Solenoceridae (5/10), Luciferidae (2/2), and Sergestidae (9/10) in two subgroups (CTFB/Decapoda | Costa et al., Biota Neotropica, 2003).
Petalidiumidae and Sicyonellidae (Vereshchaka, Royal Society Open Science, 2017) do not occur in Brazil. (33/)102 spp. in Mexico (HM).
STENOPODIDAE
(15/)105 spp. worldwide (Catalogue of Life | Bochini et al., Journal of Crustacean Biology, 2020) in 3 families (Wikipedia). All families in Brazil, with (4/)7 spp. (CTFB/Decapoda): Chicosciencea pernambucensis Bochini, Cunha, Terossi & Almeida, 2020 (Macromaxillocarididae, endemic to Brazil), Microprosthema semilaeve von Martens, 1872 (Spongicolidae, Bahamas to Bahia, Brazil), and 4 in Stenopodidae: Stenopus hispidus Olivier, 1811 from SE USA to SE Brazil, also Pacific Panama; Stenopus spinosus Risso; S. scutellatus Rankin, 1898 from SE USA to NE Brazil (Ramos-Porto & Coelho, Sér. Ci. Aquat., 1998); Odontozona lopheliae Goy & Cardoso from coast off SE Brazil, coast of SE USA, and Gulf of Mexico; and O. meloi Anker & Tavares from coast off SE Brazil, 2013 (Goy & Cardoso, Zootaxa, 2014). (2:3/)7 spp. in Mexico (HM).
PROCARIDOIDEA
A single genus and family, Procaris (5), from Mexico-1, Hawaii-1, Bermuda-1, Ascencion-1 and Christmas Islands-1. (Wikipedia).
CARIDEA
(37:456/)4,013 spp. worldwide (Catalogue of Life) — true shrimps. (24:80/)299 in Brazil (CTFB/Decapoda), in Acanthephyridae (5/17), Alpheidae (11/85), Atyidae (3/5), Barbouriidae (1/1), Bathypalaemonellidae (1/1), Campylonotidae (1/1), Crangonidae (8/10), Disciadidae (1/2), Euryrhynchidae (1/6), Glyphocrangonidae (1/8), Hippolytidae (5/8), Lysmatidae (2/12), Merguiidae (1/1), Nematocarcinidae (1/4), Ogyrididae (1/2), Oplophoridae (3/6), Palaemonidae (23/79), Pandalidae (4/19), Pasiphaeidae (4/14), Processidae (3/10), Psalidopodidae (1/1), Pseudochelidae (1/2), Rhynchocinetidae (1/2), and Thoridae (1/3). (22:106/)430 in Mexico (HM).
Agostocarididae, Alvinocarididae, Anchistioididae, Disciadidae, Gnathophyllidae, and Stylodactylidae are Mexican non Brazilian families. Acanthephyridae, Disciadidae, Campylonotidae, Euryrhynchidae, Lysmatidae, Merguiidae, Merhippolytidae, Pseudochelidae, and Thoridae are Brazilian non Mexican families.
14 families worldwide occur in freshwater, with c. 665 spp. (Balian, 2008), five of them in New World.
ALPHEIDAE ‣ mostly marine, except for a few freshwater species in the circumtropical genera Potamalpheops and Alpheus, including one Mexican freshwater species (P. stygicola Hobbs, 1973b) from two caves in northern Oaxaca [KC]. Freshwater species do not occur in Brazil (Planeta Invertebrados).
ATYIDAE ‣ (4/)10 freshwater spp. in South America. Among the five New World genera in riverine habitats, Micratya and Jonga are endemic to Caribbean up to Mexico, Costa Rica and Panama (Torati et al., ChekList, 2011); Typhlatya (17) is widely in France, Spain, Ascension, Caribbean region, Yucatan, Bermuda and Galapagos (Wikipedia); Potimirim and Atya occur in mainland South America, both in Brazil (2 and 3 spp. in country, respectively, Planeta Invertebrados). (4/)11 in Mexico [KC].
EURTRHYNCHIDAE ‣ (3/)9 rare shrimps, with Euryrhynchus (7) in South America from Colombia to French Guiana, Brazil (6, 3 endemic)and Peru (Gurgel, Dissertation, 2016), and two monotypics Euryrhynchina and Euryrhynchoides from W Africa.
PALAEMONIDAE ‣ ten freshwater genera in New World, being (4/)53 spp. in South America (Cryphiops, Macrobrachium, Palaemon and Pseudopalaemon, Planeta Invertebrados), (4/)30 of them in Brazil. In Mexico occur (6/)35 spp. in Cryphiops, Macrobrachium, Creaseria, Palaemonetes, Neopalaemon and Troglomexicanus [KC]. Troglocubanus is endemic to Cuba, and the monotypic Calathaemon (former Kakaducarididae, SEE) is endemic to Texas, USA.
XIPHOCARIDIDAE ‣ (1/)2 spp., restricted to Caribbean and Venezuela (Acta Biologica Venezuelica).
■ endemic families in New World: Anchialocarididae (1/1, Mexico).
GLYPHEIDEA
(2/)2 spp., known only by 18 specimens collected in Timor Sea, Philippines and Coral Sea (Wikipedia).
ACHELATA
(2:61/)168 spp. worldwide (Catalogue of Life), marine lobsters, in Palinuridae and Scyllaridae. (8/)14 spp. in Brazil (in both families (Scyllaridae-4/9 and Palinuridae-4/6), Cruz et al., Diversity, 2021 | CTFB/Decapoda). (2/)8 spp. in Mexico [HM].
POLYCHELIDA
(1:17/)192 living spp. worldwide (Catalogue of Life), marine lobsters, all in Polychelidae (1/4 in Brazil, no data in CTFB/Decapoda, Cruz et al., Diversity, 2021). (10/)16 spp. in Mexico [HM].
ASTACIDAE
(50/)804 spp. (Catalogue of Life) in six families, two of marine lobsters and four of freshwater crayfishes. The two lobster families are Nephropidae (14/60) and Enoplometopidae (1/10), both in Brazil (Nephropidae-3/6 and Enoplometopidae-1/1, CTFB/Decapoda). (4/)7 spp. in Mexico, in Nephropidae [HM]. (3:5/)23 spp. in Brazil.
Freshwater crayfishes includes four Astacidea families: Astacidae (4/30, 5/31 in Asia, and Pacifascatcus-8 in North America), Cambaridae (15/472, Canada to Mexico-2/56, HM), Cambaroididae (1/6, Asia), and Parastacidade (15/226, in Australia, some up to New Guinea, one in New Zealand-1/2, one in Madagascar-1/9 and 3/14 in S Chile, Argentina, Uruguay, and S Brazil in South America).
Huber (Dissertation, 2018) lists South American genera: Parastacus (18, two in Chile and 16 in Brazil, some up to NE Argentina and Uruguay, Planeta Invertebrados | Huber et al., Zootaxa, 2022), Samastacus (2, NW Argentina and C Chile) and Virilastacus (4, Chile).
AXIIDEA
(12:139/)618 spp. worldwide (Catalogue of Life). In total, Brazil has (8:24/)40 spp. (CTFB/Decapoda | Zootaxa, 2017) and Mexico has (7:22/)45 spp. [HM], numbers based on a compilation of the data below analyzed by family: Anacalliacidae (1/1 in Brazil), Axiidae (including Calocarididae, 6/11 in Brazil and 6/13 in Mexico), Callianassidae (5/10 in Brazil, 11/24 in Mexico, Mantellato et al., Zootaxa, 2022), Callianideidae (unknown in Brazil, 1/1 in Mexico), Callianopsidae (unknown in Brazil and Mexico), Callichiridae (6/11 in Brazil), Coralaxiidae (1/1 in Brazil), Ctenochelidae (3/3 in Brazil, 2/2 in Mexico), Eiconaxiidae (1/1 in Brazil, Ramos-Porto et al., Biota Neotropica, 2008, 1/4), Micheleidae (1/2 in Brazil, unknown in Mexico), Paracalliacidae (unknown in Brazil and Mexico), and Strahlaxiidae (Pacific and Indico Oceans, in New World only from Mexico-1/1 to Costa Rica).
GEBIIDEA
(3:20/)255 spp. worldwide (Catalogue of Life). Here we adopt here Coelho et al. (Zootaxa, 2017, ZY) reference for it, with complements for each family. In total, Brazil has (2:3/)11 spp. (2:3/)19 in Mexico [HM]. By family: Laomediidae (including Axianassidae, 1/1 in Brazil, 1/2 in Mexico), Thalassinidae (not recorded in Brazil or Mexico), and Upogebiidae (2/10 in Brazil, 2/17 in Mexico).
ANOMURA
23 families (20 exclusivelly marine) and (268/)3,513 spp. worldwide (Catalogue of Life). (13:50/)197 spp. in Brazil (CTFB/Decapoda), in Aeglidae (1/68), Albuneidae (4/6), Blepharipodidae (1/1), Chirostylidae (2/4), Diogenidae (10/27), Hippidae (2/3), Lithodidae (1/2), Munididae (2/18), Munidopsidae (2/11), Paguridae (14/28), Parapaguridae (3/6), Porcellanidae (7/22), and Pylochelidae (1/1). (14:75/)340 in Mexico [HM]. Mexican families Galatheidae and Coenobitidae do not occur in Brazil. Brazilian family Aeglidae does not occur in Mexico.
Only three lineages if Anomura are found in freshwater envioronments: Aeglidae (1/87), Clibanarius fonticola McLaughlin & Murray, 1990 endemic to Vanuatu (Espírito Santo island, in a pool fed by springs near the village of Matevulu, Wikipedia), and Coenobitidae (15), with two spp. in New World, Coenobita compressus H. Milne-Edwards from Pacific coast up to Chile (Wikipedia), and C. clypeatus Fabricius from Caribbean region, Colombia and Venezuela (Wikipedia), both not recorded in Brazil (Lemaitre & Tavares, Zootaxa, 2015).
Among Aeglidae, Aegla is the only extant genus, containing 87 spp. from S Bolivia, Chile, Argentina, S & SE Brazil (55), Paraguay and Uruguay, two are found exclusively in lakes (both in Chile), four troglobics (all in SE Brazil, unique troglobic Decapoda in country), and the remaining 81 are found mainly in rivers (Trombetta et al., Zootaxa, 2019).
BRACHYURA (CRAB DATABASE)
(122:1,683/)8,050 living spp. of true crabs worldwide (Catalogue of Life), (51:210/)410 in Brazil (CTFB/Decapoda), in Acidopsidae (1/1), Aethridae (2/5), Belliidae (2/2), Calappidae (4/11), Carcinidae (1/1), Carpiliidae (1/1), Chasmocarcinidae (1/7), Cryptochiridae (3/3), Cyclodorippidae (4/8), Cymonomidae (2/4), Domeciidae (1/1), Dromiidae (4/5), Epialtidae (20/29), Eriphiidae (1/1), Ethusidae (2/4), Euryplacidae (2/2), Gecarcinidae (2/2), Geryonidae (1/3), Goneplacidae (2/2), Grapsidae (5/7), Homolidae (2/3), Homolodromiidae (2/3), Hymenosomatidae (3/3), Inachidae (7/9), Inachoididae (11/17), Latreilliidae (1/1), Leucosiidae (9/20), Majidae (13/35), Mathildellidae (1/2), Menippidae (1/1), Ocypodidae (3/12), Palicidae (1/7), Panopeidae (11/21), Parapinnixidae (1/2), Parthenopidae (13/17), Pilumnidae (1/7), Pilumnoididae (1/3), Pinnotheridae (11/16), Plagusiidae (3/5), Platyxanthidae (1/2), Polybiidae (1/1), Portunidae (9/25), Pseudorhombilidae (2/3), Pseudothelphusidae (5/20), Pseudoziidae (1/1), Raninidae (3/5), Sesarmidae (3/7), Trichodactylidae (10/33), Trichopeltariidae (2/2), Varunidae (3/5), and Xanthidae (14/23).
(51:272/)740 in Mexico [HM]. Mexican families Dynomenidae, Bythograeidae, Cancridae, Oregoniidae, Atelecyclidae, Trapeziidae, Hexapodidae, Goneplacidae, Oziidae, Ucididae and Glyptograpsidae do not occur in Brazil. Brazilian families Belliidae, Cymonomidae, Platyxanthidae, Latreilliidae, Hymenosomatidae, Carcinidae, Geryonidae, Pseudoziidae and Trichopeltariidae do not occur in Mexico.
For freshwater crabs, eight families are known, only two in New World, Trichodactylidae and Pseudotelphusidae, with 311 spp. in region (Cumberlidge et al., ZooKeys, 2014). Colombia (21/105) has the largest diversity (Pseudothelphusidae-15/90, six endemic genera, and Trichodactylidae-9/15). Mexico has the second diversity in region (16/67 spp., all endemic, in Pseudothelphusidae-14/61, 11 endemic genera, Trichodactylidae-2/5, Moreno-Juaréz, Zootaxa, 2022). Brazil has the 3rd diversity in freshwater crabs (17/56, 17 endemic, Planeta Invertebrados) in New World, in Trichodactylidae (10/28, in two subfamilies) and Pseudothelphusidae (7/28 species, all Pseudothelphusinae/Kingsleyini). Brazil has 2✕ as many Trichodactylidae as Colombia, which in turn has more than 3✕ more Pseudotelphusidae tham our country. In 2020 (by Campos et al., Nauplius, 2020) Colombia was considered the most species-rich country for freshwater crabs (109, 86 endemic) in South America and the second worldwide after China (243). Trichodactylus (4) is endemic to Brazil (Souza-Carvalho, E. et al., Arthropod Systematics & Phylogeny, 2025).
Freshwater crabs do not occur in USA (DFO/MPO).
■ endemic families in New World: Ectaesthesiidae (1/1, Ecuador).
ORDER AMPHIPODA
(254:1,916/)11,040 spp. worldwide (Catalogue of Life) and (73:183/)392 spp. in Brazil (CTFB/Amphipoda | Magnovidae | SB/2013). Serejo e Siqueira (Zootaxa, 2018) lists (56:142/)303 spp. distributed in the suborders Amphilochidea, Senticaudata and Ingolfiellida that have been described or reported for the Brazilian coast and continent.
Six suborders make up order Amphipoda, 4 in Brazil: Amphilochidea, Colomastigidea (known in Brazil only for Colomastix trispinosa Alves & Senna, 2019, Novataxa), Hyperiidea and Senticaudata — and two not recorded: Hyperiopsidea (2:5/15, widely worldwide/Copepedia, present in Mexico/RBT) and Pseudingolfiellidea (1:1/4, freshwater known only from southern South America and some subantarctic islands, Marine species).
Senticaudata includes the only family of Amphipoda with terrestrial representatives, Talitridae (84/257, SEE), with four spp. along the Brazilian coast: the three Brazilian endemic Chelorchestia darwinii Müller, 1864, Atlantorchestoidea brasiliensis Dana, 1853 and Talorchestia tucurauna Müller, 1864; and the Atlantic Platorchestia monodi Mateu et al., 1986 (Serejo, Zootaxa, 2004).
Aproximatelly 1,870 spp. in freshwater (Balian, 2008), in 53 families and 293 genera, but only 27 families are strictly limited to continental waters. Currently five families of amphipods are known for subterranean ecosystems in Brazil (PBSW | Wikipedia | SB/2013 | UFRN | Nauplius | Zootaxa): Artesiidae (2/10, Artesia-2 in Texas, USA, and Spelaeogammarus-8 in Brazil), Bogidiellidae (one freshwater genus, Megagidiella), Hyalellidae (1/81, Canada to Patagonia, 44 in Brazil), Mesogammaridae (Korea, Japan, Indian subcontinent, southern Alaska and caves from Rio Grande do Norte state in NE Brazil), and Seborgidae (Vietnan, islands in adjacences of Papua New Guinea, Texas and caves from from Rio Grande do Norte state in NE Brazil). Hyalella (42) is the only known freshwater Amphipoda genus in Brazil to occur in superficial environments (Deotti, CM et al., Nauplius, 2025).
Besides the five families occurring in freshwater in Brazil, another four are reported for South America (Balian, 2008 | C. Fiser et al., Systematics and Biodiversity, 2013 | Schultheiss, Zootaxa, 2013): Pseudingolfiellidae (New Zealand, Chile and Crozet Island, 1/3, Smet, Zootaxa, 2015), Falklandellidae (2/2, Chile and Falkland islands), Corophiidae (Balian, 2008 | Invasions), Phreatogammaridae (New Caledonia, New Zealand and Chile, Bréhier et al., Crustacean Biology, 2010), and Paraleptamphopidae (New Zealand and Chile, Grosso & Peralta, Zootaxa, 2009). All families except Falklandellididae and Hyalellidae have a transoceanic distribution and South American members are found only from subterranean waters.
Hyalella imbya Rodrigues & Bueno, 2012, known only in a small area in W Rio Grande do Sul state in southern Brazil, is the first hypothelminorheic Arthropoda from South America (Stella Gomes Rodrigues et al., ZooKeys, 2012).
■ endemic families in New World: Allocrangonyctidae (1/2, USA), Parabogidiellidae (2/2, USA) and Magnoviidae (1/1, Brazil).
ORDER STYGIOMYSIDA
16 spp. in two families. Lepidomysidae has 9 spp., all in Spelaeomysis (Lucio Pesce), being S. villalobosi (Mexico, Nuevo Leon, SEE), S. quinterensis (Mexico, Tamaulipas and San Luis Potosí), S. olivae (Mexico, Oaxaca), S. nuniezi (Cuba), S. cardisomae (San Andrés island, Colombia), S. bottazzii (S Italy), S. servatus (Kenya, Tanzania and Aldabra), S. longipes (Kerala, India) and S. cochinensis (Kerala, S India). Stygiomysidae has 7 spp., all in Stygiomysis (Lucio Pesce), being S. cokei (Quintana Roo, Mexico, SEE, S. aemete (República Dominicana, SEE), S. clarkei (Turks and Caicos Islands), S. holthuisi (St. Martin, Anguilla, Puerto Rico and Grand Bahama), S. ibarrae (Cuba, SEE), S. major (Jamaica) and S. hydruntina (S Italy).
Stygiomysida has traditionally been considered part of the order Mysida, but was separated from it on phylogenetic grounds. Unknown in Brazil.
ORDER MYSIDA
Two families worldwide, Mysidae and Petalophthalmidae, with (197/)1,245 spp. (Catalogue of Life). (14/)30 spp. in Brazil (CTFB), all in Mysidae.
Approximately 80 are freshwater, nine in Brazil, in two genera: Parvimysis and Surinamysis (Planeta Invertebrados). 23 spp. occur in Mexico (Bousquets et al.,Conabio, 2000).
ORDER TANAIDACEA
(42:346/)1,634 spp. worldwide (Catalogue of Life), (17:46/)62 spp. in Brazil (CTFB/Tanaidacea). Only four in freshwater: one in Baikal Lake, two in Oceania, and Sinelobus stanfordi Richardson, 1901, a marine, freshwater, hypohaline and hypersaline species inland waters of Galapagos, Japan, Hong Kong, New Zealand, Australia, Argentina, Kurile Islands, Caribbean, Florida and Brazil (Balian, 2008 | Planeta Invertebrados). 24 spp. in Mexico (Bousquets et al., Conabio, 2000).
ORDER CUMACEA
(8:201/)1,863 spp. worldwide (Catalogue of Life), (6:34/)98 spp. in Brazil (CTFB/Cumacea). 32 spp. in Mexico, being eigth in Gulf of Mexico, 11 in Caribbean Sea and 13 in Pacific Ocean (Bousquets et al., Conabio, 2000).
All species are marine except (11/)21 spp. in freshwater, all Pseudocumatidae from Caspian basin except two New World species, in Nannastacidae: Almyracuma proximoculi Jones and Burbanck, 1959 from intertidal freshwater springs at Cape Cod, and limnetic zone of Lower Hudson river, NE USA, and Claudicuma platense Roccatagliata, 1981 from Río de la Plata (Argentina), from Buenos Aires to Punta del Indio (Roccatagliata, Marine Biodiversity, 2017).
ORDER ISOPODA
(144:1,624/)11,268 spp. worldwide (Catalogue of Life), with around 4,500 spp. found in sea, mostly on the seabed, 500 in freshwater, and more than 5,000 on land (Wikipedia). Brazil includes (60:176/)490 spp., being (57:170/)481 spp. in CTFB/Isopoda plus two families of Calabozoidea (6/8 spp. in country, Brazilian Cave Fauna) and Limnoriidae (at least 1 sp., SEE).
All suborders in Isopoda occur in Brazil except Phoratopidea (continental shelf at Encounter Bay and Fowlers Bay, South Australia, Wikipedia), Phreatoicidea (freshwater environments in South Africa, India, and Oceania, SEE) and Tainisopidea (Tainisopidae, 2/7, endemic to Australia, Intreasures).
■ endemic families in New World: Brasileirinidae (1/2, Brazil).
ORDER THERMOSBAENACEA
(4:8/)45 spp. worldwide (Catalogue of Life). Thermosbaenidae includes a single species from saline hot springs from Tunisia. Tulumellidae includes (1/)3 spp. from anchialine caves in SE Mexico (1) and Bahamas (2). In Halosbaenidae there are four genera: Salishbaena (1, Montana, USA, SEE) and Theosbaena (3, in Thailand-2 and Cambodia-1) are fully troglobics; Limnosbaena (2) are freshwater species in wells and lakes from Bosnia, Italy and S France; Halosbaena (5) includes three marine-cave species from S Japan-2 and Canary Island-1, one coastal marine species in Curaçao, Bonaire, Venezuela, San Andres Island in Colombia, Cuba and Jamaica, and H. tulki, a troglobic from W Australia. Monodellidae includes two genera: Monodella (1) in oligohaline caves and wells near coast in NW Italy; and Tethysbaena (29) widely widespread in Texas (1), Cuba (3), Haiti (2), República Dominicana (2), Puerto Rico (2), British Virgin Island (3), US Virgin Islands (1), Spain (2), France (2), Italy (2), Morocco (1), Croatia (1), Greece (1), Somalia (1), Israel (1), Oman (3), and Socotra (1). Unknown in Brazil.
ORDER MICTACEA
A single family, genus and species (Catalogue of Life), Mictocaris halope Bowman & Iliffe, 1985, endemic to anchialine caves in Bermuda, and grows up to 3.5 mm long (Wikipedia). Unknown in Brazil.
■ endemic families in New World: Mictocarididae (1/1, Bermudas).
ORDER BOCHUSACEA
A single family, Hirsutiidae, possibly a part of a wider Mictacea, and six species in three genera (Catalogue of Life): Hirsutia bathyalis Saunders, Hessler & Garner, 1985 (depth of 1000 m at 300 km off coast of Suriname, Sanders et al., Journal of Crustacean Biology, 1985), H. sandersetalia Just & Poore, 1988 (depth of 1,500 m in the South Pacific, off SE Australia), Thetispelecaris remex Gutu & Iliffe, 1998 (anchialine and marine caves in the Exuma Cays, Bahamas), T. yurikago Ohtsuka, Hanamura & Kase, 2002 (in submarine cave on Grand Cayman Island), T. kumejimensis Shimomura, Fujita & Naruse, 2012 (Japan, SEE), and Montucaris distincta Jaume, Boxshall & Bamber, 2006 (619–778 m depth off coast of SE Brazil, D. Jaume et al., ZJLS, 2006).
ORDER LOPHOGASTRIDA
(3:10/)53 spp. worldwide (Catalogue of Life). In Brazil at least two species and families was collected: Neognathophausia ingens Dohrn, 1870 in Lophogastridae, and Gnathophausia zoea Willemoes-Suhm, 1873 in Gnathophausiidae (Serejo et al., BOOK, 2007), from Bahia to Rio Grande do Sul. Few informations about Eucopiidae (10). Unknown in Mexico (Bousquets et al., Conabio, 2000).
ORDER INGOLFIELLIDA
(5/)46 spp. worldwide in two families, Metaingolfiellidae and Ingolfiellidae (Wikipedia | no data in Catalogue of Life), 5 in South America, all in latter family (Rodriguez, M. et al., Zootaxa, 2017): Ingolfiella uspallatae Noodt, 1965 (Argentina), I. manni Noodt, 1961 (Chile), I. ruffoi Siewing, 1958 (Peru, SEE), I. rocaensis Senna & Serejo, 2005 (unique record of Ingolfiellida in Brazil, from Rocas Atoll, Senna & Serejo, Zootaxa, 2005 | CTFB/Malacostraca), and Yacana ventania Rodriguez, Armendáriz & Rodrígues Capítulo, 2017 (E Argentina). Ingolfiella (35) has two spp. in Gulf of Mexico (LAJAR).
ORDER SPELEOGRIPHACEA
(3/)4 fully freshwater species in a single family (Catalogue of Life), Spelaeogriphidae: Potiicoara in WC Brazil, Mangkurtu in W Australia and Spelaeogriphus in South Africa (Balian, 2008). Brazilian member is the obligatory subterranean species Potiicoara brasiliensis Pires, 1987, known only caves from SW Mato Grosso and W Mato Grosso do Sul states.
29.10 CEPHALOCARIDA CLASS ‣ small class composed only of a single family in its own order, Hutchinsoniellidae, with (5/)12 spp. (Addis, Thesis, 2008): Chiltoniella (1, New Zealand), Hampsonellus (1, Brazil, restricted to the coast of São Paulo), Hutchinsonella (1, disjunct SE Brazil and E USA), Lightiella (5, coasts from Canada to Florida, Mexico, Caribbean, Mediterranean and New Caledonia) and Sandersiella (4, Korea, Japan, Namibia and Peru).
29.11 BRANCHIOPODA CLASS ‣ three orders with (34:172/)1,847 spp. worldwide and (19:74/)189 spp. in Brazil, numbers based on a compilation of the data below analyzed by taxonomic group. Maeda-Martínez et al. (Hydrobiologia, 1997) cites 143 spp. in Mexico, in all groups: Anostraca (20), Spinicaudata (11), Cyclestherida (1), Cladocera (106), Laevicaudata (2) and Notostraca (3), although some of these numbers are more up to date.



ORDER ANOSTRACA
(9:34/)372 spp. on all continents, including Antarctica (Catalogue of Life). Brazil has (3:5/)15 spp. (7/)53 spp. in freshwater Neotropics (Rogers, 2020), in 4 families: Artemiidae (1/2, 1 in Brazil), Branchinectidae (1/19, 2 in Brazil), Streptocephalidae (1/8 in Neotropics, all from Mexico and Caribbean, also in North America and Old World) and Thamnocephalidae (4/24, 3/12 in Brazil, Phallocryptus is endemic to Argentina). CTFB recognizes only 8 Thamnocephalidae in Brazil (CTFB/Branchiopoda).
ORDER DIPLOSTRACA
(24:136/)1,452 spp. worldwide in three suborders, (16:69/)174 in Brazil, numbers based on a compilation of the data below analyzed by group.
SPINICAUDATA
(18/)259 spp. in four families: Eocyzicidae (2/34), Cyzicidae (3/76 — 1/4 in Neotropics, in Brazil (2), Mexico and Cuba), Leptestheriidae (3/40 — 1/5 in Neotropics, in Venezuela, Mexico, Peru, Bolivia, Chile, Argentina and Brazil-1, Rogers 2020 | Weddingen and Rabet, Zoological Studies, 2020), and Limnadiidae (10/109 — 2/9 in Neotropics, widely in several areas, five Eulimnadia in Brazil). Three families in Brazil, with (3/)8 spp. (CTFB/Branchiopoda).
CLADOCEROMORPHA
(19:115/)1,150 spp. (Catalogue of Life, includes Cyclestherida), (9:50/)186 spp. in Neotropics (Balian, 2008). Brazil has (12:65/)165 spp. of Cladoceromorpha (CTFB/Branchiopoda), in four groups: Anomopoda, Ctenopoda, Cyclestherida and Onychopoda. (7:33/)106 spp. in Mexico (Bousquets et al., Conabio, 2000).
LAEVICAUDA
Only a single family, Lynceidae, with (3/)43 spp. worldwide (Catalogue of Life). (2/)8 spp. in Neotropics (Rogers, 2020), collected from Mexico (2/7, KNF/2016; KNAF/2020) and South America, 7 in this continent (Pessac et al., Zootaxa, 2011 | Fonseca et al., Wetlands, 2017 | Sigvardt et al., Invertebrate Systematics, 2019): Lynceus aequatorialis Daday,1927 (Colombia and Venezuela), L. tropicus Daday, 1927 (Venezuela), L. rotundirostris Daday, 1902 (S Argentina), L. mallinensis (S Argentina), L. huentelauquensis (Chile), Paralimnetis mapimi (Mexico, Colombia), P. rapax Gurney, 1931 (Colombia, Paraguay and Brazil), the latter record not listed in the CTFB in Feb 08, 2026.
ORDER NOTOSTRACA
Only a single family with (2/)23 spp. worldwide in Triops and Lepidurus (Catalogue of Life). (2/)2 spp. in Neotropics, collected in Mexico (2/2, KNAF/2020; KNF/2016), Venezuela, Ecuador, Bolivia, Argentina and Caribbean (Rogers, 2020), unknown in Brazil.
■ endemic families in New World: Dumontidae (1/1, USA).
29.12 COPEPODA CLASS ‣ (253:2,479/)15,665 spp. worldwide in two infraclasses and current 10 orders, and (78:290/)867 spp. in Brazil, numbers based on a compilation of the data below analyzed by order. In Mexico occur (7:31/)78 spp. in freshwater and (50:122/)479 in marine environments (Bousquets et al., Conabio, 2000). Copepoda includes ten orders, six of which (Platycopioida, Calanoida, Misophrioida, Cyclopoida, Harpacticoida, Gelyelloida) contain stygobiotic representatives.




INFRACLASS PROGYMNOPLEA
ORDER PLATYCOPIOIDA
(4/)11 spp. in a single family Platycopiidae (Catalogue of Life), from Bermudas, Barents Sea, North Sea, E North America, Bahamas, Mauritania and Japan (Wikipedia), not recorded in Brazil.
INFRACLASS NEOCOPEPODA (9 ORDERS)
GYMNOPLEA/CALANOIDA
(45:393/)2,873 spp. worldwide (Catalogue of Life), (28:97/)319 spp. in Brazil (CTFB/Copepoda). Calanoida are rare in fresh ground water, represented only by 10 species in the family Diaptomidae, all of which lead a planktonic mode of life in subterranean lakes.
PODOPLEA/ORDER MISOPHRIOIDA
(3:19/)37 spp. worldwide (Catalogue of Life). Only one sp. in Brazil, Archimisophria squamosa Alvarez, 1985, record not listed in the CTFB in February 08, 2026, but accepted here (Alvarez, MPJ., Journal of Natural History, 1985). Almost all Speleophriidae species occur in anchialine coastal habitats, the only exceptions being the two species of Archimisophria, both of which occur in the deep hyperbenthic community in the tropical Atlantic (Boxshall, G.A., Zootaxa, 2014).
PODOPLEA/ORDER CYCLOPOIDA
(103:866/)4,979 spp. (Catalogue of Life), (9/69:)259 spp. in Brazil (CTFB). Cyclopoida are groundwater copepods par excellence, represented by over 330 stygobiotic species in the family Cyclopidae.
PODOPLEA/ORDER GELYELLOIDA
(1/)2 spp. in groundwater in saturated karstic areas of S France and W Switzerland (Catalogue of Life | Wikipedia); no published records are available from surface habitats.
PODOPLEA/ORDER MORMONILLOIDA
(3/)5 spp. in a single family, Mormonillidae (Catalogue of Life), widely in Atlantic Ocean, Pacific and Meditteraneam Basin (WoRMS), including records of Mormonilla phasma Giesbrecht, 1891 in Rio Grande do Norte state, NE Brazil (Araújo & Neumann-Leitão, Annals of the Brazilian Academy of Sciences, 2015), record not listed in the CTFB in February 08, 2026, but accepted here.
PODOPLEA/ORDER HARPACTICOIDA
(56:770/)5,006 spp. worldwide (Catalogue of Life), (23:65/)139 in Brazil (CTFB/Copepoda, excludes current Canuelloida/Longipediidae).
Bernot, J.P. et al. (Molecular Phylogenetics and Evolution, 2025) recognizes Canuelloida (two families, Canuellidae and Longipediidae) as a valid order separate from Harpacticoida. Harpacticoida are groundwater copepods par excellence, containing some 640 truly freshwater stygobionts belonging to at least five families. Families Canthocamptidae, Parastenocarididae and Ameiridae have radiated very successfully in groundwater habitats. Sporadically, members of the predominantly marine Ectinosomatidae and Miraciidae (formerly Diosaccidae) are recorded from fresh ground water. The Chappuisiidae are only known from Europe, being exclusively found in alluvial aquifers and in the hyporheic habitat. The Phyllognathopodidae are occasionally found in ground water.
PODOPLEA/ORDER CANUELLOIDA
(2:19/)91 spp. (Catalogue of Life) in Canuellidae (18/67) and Longipediidae (1/24). In Brazil occurs only one genus of Canuelloida, Longipedia, with six species in country (CTFB).
PODOPLEA/ORDER SIPHONOSTOMATOIDA
(40:396/)2,459 spp. worldwide (Catalogue of Life), (14:52/)128 spp. in Brazil (CTFB).
PODOPLEA/ORDER MONSTRILLOIDA
A single family, (8/)202 spp. worldwide (Catalogue of Life), (4/)14 in Brazil (Suárez-Morales, E. et al., Zoologia, 2024 | CTFB/Copepoda).
29.13 REMIPEDIA CLASS ‣ (8:12/)28 spp. within a single order Nectiopoda (Remipedia Database) in Caribbean, Mexico, Belize, Canary Islands and NW Australia.
By family: Cryptocorynetidae (3/5, Bahamas/4 and Turks y Caicos/1), Godzilliidae (2/4, Bahamas and Turks y Caicos), Micropacteridae (1/1, Turks y Caicos), Pleomothridae (1/2, Bahamas), Speleonectidae (2/7, Bahamas, Turks y Caicos and SW Cuba in southern Matanzas coast), Xibalbanidae (1/4, anchialine caves in Caribbean coast of SE Mexico/3 and Belize/1), Kumongidae (1/1, Ningaloo Cape in NW Australia, unique southern Hemisphere species) and Morlockiidae (1/4, Bahamas/2, SE República Dominicana/1 and Lanzarote in Canary Islands in Spain/1).
By country: Bahamas (7/16), Turks y Caicos (4/4), SE Mexico (1/3), Australia (1/1), Belize (1/1), Cuba (1/1), República Dominicana (1/1) and Canary Islands (1/1).
■ endemic families in New World: Pleomothridae (1/2, Bahamas) and Micropacteridae (1/1, Turks y Caicos).
29.14 COLLEMBOLA CLASS ‣ (33:776/)9,092 spp. worldwide (Catalogue of Life) and (21:131/)618 spp. in Brazil, belonging all four known orders (CTFB/Collmebola | do not include new cave species, which can be seen in Brazilian Cave Fauna). Colombia has (16:21/)38 spp. (Cipola, Zootaxa, 2023). (24:105-107/)582 spp. in Mexico (Palacios-Vargas, Revista Mexicana de Biodiversidad, vol. 85, 2014). An illustrated guide with photos of almost every family in the order is available at Checklist of the Collembola (SEE).
Mexican families Poduridae, Actaletidae, Coenaletidae and Tomoceridae do not occur in Brazil. Brazilian Sturmiidae does not occur in Mexico. In following families Mexico certainly has more species than Brazil: Hypogastruridae (80 ✕ 30), Odontellidae (16 ✕ 1), Onychiuridae (21 ✕ 4) at Poduromorpha; Oncopoduridae (5 ✕ 2) in Entomobryomorpha; Katiannidae (8 ✕ 3), Bourletiellidae (10 ✕ 9) and Dicyrtomidae (15 ✕ 4) in Symphypleona; and Neelidae in Neelipleona (10 ✕ 3).
ORDER PODUROMORPHA
(11:372/)3,591 spp. (Catalogue of Life), (7:49/)138 spp. in Brazil, in Neanuridae (22/70), Hypogastruridae (11/30), Brachystomellidae (8/24), Tullbergiidae (3/7), Onychiuridae (3/4), Isotogastruridae (1/2) and Odontellidae (1/1). Gulgastruridae, Pachytullbergiidae, Paleotullbergiidae, and Poduridae do not occur in Brazil.
ORDER ENTOMOBRYOMORPHA
(11:271/)4,271 spp. (Catalogue of Life), (5:57/)390 spp. in Brazil, in Entomobryidae (19/179), Isotomidae (22/80), Paronellidae (10/105), Orchesellidae (5/20) and Oncopoduridae (1/6). Actaletidae, Coenaletidae (1/2, New Guinea, Guadaloupe, Mexico, Dominican Republic, Colombia, AESA | Collembola | CS/2025), Oncobryidae, Praentomobryidae, Protentomobryidae, and Tomoceridae does not occur in Brazil.
ORDER SYMPHYPLEONA
(10:125/)1,175 spp. (Catalogue of Life), (8:23/)87 spp. in Brazil, in Sminthuridae (7/30), Sminthurididae (4/28), Bourletiellidae (5/10), Arrhopalitidae (1/9), Dicyrtomidae (2/4), Katiannidae (2/3), Collophoridae (1/2) and Sturmiidae (1/1). Mackenziellidae and Spinothecidae do not occur in Brazil.
Sphaeridia pilleata Bretfeld and Gauer, 1994 (Sminthurididae) from Brazil, is arguably the smallest known adult hexapod, with its smaller males measuring about 0.12 mm (Bellini, Weiner & Winck, Diversity, 2023).
ORDER NEELIPLEONA
A single family, Neelidae, with (8/)55 spp. worldwide (Catalogue of Life), (2/)3 in Brazil, including Megalothorax minimus V.Willem, 1900 from Pará and Neelus minimus Womersley, 1932 from Espírito Santo states.
29.15 PROTURA CLASS ‣ (7:77/)821 spp. worldwide in three orders (Catalogue of Life | Galli et al., ZooKeys, 2018). Brazil has (2:13/)27 spp. (CTFB/Protura). (2:6/)17 spp. occur in Mexico (Palacios-Vargas & Figueroa, Revista Mexicana de Biodiversidad, vol. 85, 2014).
ORDER ACERENTOMATA
(64/)458 spp. worldwide (Catalogue of Life) in three families: Hesperentomidae (3/28) from in East and Central Asia, Europe and North America, Protentomidae (6/44) in Japan, China, Pacific Islands, tropical Asia, Reunion, North America and Argentina (see Vidal Sarmiento, Physis, 1971 for South America), and Acerentomidae (55/386), with (11/)14 spp. in Brazil and (4/)6 in Mexico.
ORDER SINENTOMATA
(2:2/)5 spp. (Catalogue of Life) in Fujientomidae and Sinentomidae, known only from China, Japan and North Korea.
ORDER EOSENTOMATA
(11/)358 spp. worldwide (Catalogue of Life) in two families, Antelientomidae (one genus, endemic to China) and Eosentomidae with 10 genera, six restricted from China and Japan, Styletoentomon from North America, Madagascarentomon endemic to Madagascar, and Isoentomon and Eosentomon widely distributed in World, both in Brazil (2/13) and in Mexico (2/11).
29.16 DIPLURA CLASS ‣ three superfamilies (all in Brazil) and (10:143/)866 spp. worldwide, but no formal order, numbers based on a compilation of the data below analyzed by order (Catalogue of Life | Sendra, Insect Conservation and Diversity, 2021), (4:15/)31 in Brazil (CTFB/Diplura). South America has (35/)154 spp., Mexico has (6:17/)48 spp. (Palácios-Vargas & García-Goméz, Revista Mexicana de Biodiversidad, 2014), and USA has (7:23/)170 spp.
CAMPODEOIDEA
(61/)349 spp. worldwide (Catalogue of Life) in two families: Procampodeidae (1/2, Italy and California one each, Wikipedia) and Campodeidae (60/347, widely worldwide). (1:5/)5 spp. in Brazil (CTFB/Diplura) and (9/)33 in Mexico.
JAPYGOIDEA
(75/)467 spp. worldwide (Sendra, Insect Conservation and Diversity, 2021, data on Catalogue of Life inconclusive), in Japygidae (61/340) widely worldwide, Evalljapygidae (5/47, 34 in W North America and 12 in South America), Parajapygidae (4/66,Parajapyx-55 worldwide, Ectasjapyx-1 in C Africa), Miojapyx-11 in USA, and Lacandonajapyx-1 in Mexico — Montejo-Cruz, Zootaxa, 2021), Heterojapygidae (4/10, Australia and Madagascar) and Dinjapygidae (1/6, Peru and Bolivia).
(7/)15 spp. in Brazil (CTFB/Diplura), in Japygidae (6/12) and Parajapygidae (1/3), and (7/)21 in Mexico (Japygidae, Parajapygidae and Evalljapygidae).
The circumscription of Evalljapygidae varies from reference to reference, with Sendra (Insect Conservation and Diversity, 2021) citing 5 genera without specifying which ones, and indicating (3/)12 spp. in South America. Wikimedia (SEE) lists only 3 genera, with Mixojapyx being cited for Brazil (Figueredo, Dissertation, 2009). Gracía-Goméz (BSEA, 2010) cites only two, none of them in South America, a position reinforced by Allen (TAES, 2002) at subfamily Evalljapyginae. Therefore, we adopted a very conservative version, aligned with the last two references.
PROJAPYGOIDEA
(7/)50 worldwide (Sendra, Insect Conservation and Diversity, 2021, no data in Catalogue of Life), in Anajapygidae (2/5, Holartic, Oriental, Neotropical, 2 in Mexico), Octostigmatidae (1/3, Asia and Australia), and Projapygidae (4/42, widely worldwide). (1:3/)11 spp. in Brazil (CTFB/Diplura) and (1/)2 in Mexico.
There is an isolated record of Anajapygidae from caves in Espírito Santo state, SE Brazil (Silva & Ferreira, Subterranean Biology, 2015), but it has not been formally described and is therefore not accepted here.
29.17 INSECTA CLASS ‣ (1,445:114,915/)1,077,159 spp. worldwide and (703:15,939/)91,241 spp. in Brazil, numbers based on a compilation of the data below analyzed by order. These numbers may be far from the real values, either because insect diversity remains largely unknown, because current knowledge is highly fragmented, or due to methodological errors in data compilation. These insects are distributed across 28 orders. Two of these orders do not occur in Brazil (Notoptera and Rhaphidioptera), which harbors an immense diversity of species but shows a low representation of certain groups, such as Plecoptera, as well as significant gaps in some lineages, particularly within Lepidoptera.
1 ARCHAEOGNATHA
(65/)548 spp. worldwide in two families (Foottit & Adler, vol. 2, 2009, pg. 156). Machilidae has (38/)250 spp. worldwide, only nine genera in New World, from Canada to Mexico (Foottit, 2018). Meineterllidae has (19/)170 spp. worldwide (Wikipedia), (2/)25 spp. in Brazil (CTFB/Insecta). Four of six South American genera are continentally confined to Argentina and Chile (Rafael, 2012). Mexico has (2:9/)15 spp. in this order, being (5/)7 spp. in Machilidae and (4/)8 spp. in Meineterllidae (Palacios-Vargas, BOOK, 2004), and USA has (14/)36 spp., being (10/)27 in Machilidae and (4/)9 in Meineterllidae (Bristletails of North America).
Some unconfirmed records of Machilidae in Brazil (as in FLICKR) in Brazil are not considered here.
2 ORDER ZYGENTOMA
(149/)594 spp. wordlwide in five families (Foottit & Adler, vol. 2, 2009, pg. 156), only Nicoletiidae (15/22) and Lepismatiidae (6/10) in Brazil, with (21/)32 spp. in the country (Rafael et al., BOOK, 2024).
Remaining families are Lepidotrichidae (1/1, endemic to USA), Maindroniidae (5, 3 in Sudan and Arabian Peninsula, Atacamus-1 in Chile, and Peruatacamus-1 in Peru — Irish et al., Zoologischer Anzeiger, 2025), and Protrinemuridae (4/10 spp., worldwide, only two in New World, both Trinemophora collected in W South America — Foottit/2018).
Some unconfirmed records of Lepidotrichidae in Brazil (as in CBE/2009) in Brazil are not considered here.
■ endemic families in New World: Lepidotrichidae (1/1, USA).
3 ORDER EPHEMEROPTERA
(48:560/)3,813 spp. worldwide (Catalogue of Life), (10:81/)478 in Brazil (CTFB/Ephemeroptera), including one endemic family.
■ endemic families in New World: Melanemerellidae (1/1, Brazil).
4 ORDER ODONATA
(58:902/)6,518 spp. worldwide (Catalogue of Life | Families and Genera of Odonata) in three suborders: Anisoptera (dragonflies, 21:503/3,150), Zygoptera (damselflies, 36:398/3,365), and the intermediate Anisozygoptera (1:1/3, Indian subcontinent, E Asia, and SE Asia, Wikipedia).
In Brazil occur (18:149/)910 spp., in Aeshnidae (10/62), Gomphidae (22/120), Aeschnosomatidae (2/9), Corduliidae (3/11), Idomacromiidae (1/12), Lauromacromiidae (1/6), Libellulidae (38/238) in Anisoptera (7:77/458); and Calopterygidae (3/37), Coenagrionidae (54/331), Dicteriadidae (2/2), Heteragrionidae (2/36), Lestidae (2/16), Megapodagrionidae (2/3), Perilestidae (2/12), Philogeniidae (1/2), Platystictidae (1/1), Polythoridae (2/11), Rimanellidae (1/1) in Zygoptera (11:72/452), among all South American families except Neopetaliidae (1, known only from Argentina and Chile, Wikipedia), Petaluridae (5/11, E Asia, Australia, New Zealand, North America, Argentina and Chile, SEE), Mesagrionidae (1/1, endemic to Colombia), and Austropetaliidae (Argentina, Chile and Australia).
The continental countries with highest richness of Odonata are Venezuela (548) and Colombia (543 species). Brazil (863 or 910) and China (818) have higher richness, but given their size were included in the analysis as states and regions. Excluding very large islands (New Guinea, Sumatra or Borneo), which are considered continents in this paper, Japan (209) is the archipelago with highest richness, albeit Indonesia (737) and the Philippines (306) have more species, but were analysed subdivided by islands (Cordero-Rivera, PeerJ, 2025).
■ endemic families in New World: Mesagrionidae (1/1, Colombia).
5 ORDER ORTOPTERA
(43:5,068/)29,744 spp. worldwide and (19:581/)1,855 in Brazil, numbers based on a compilation of the data below analyzed by group, in two clades, Ensifera and Caelifera. (274/)c. 920 spp. in Mexico (Insecta/MX). The classification of the groups adopted here follows Jasso-Martínez, JM et al (Molecular Phylogenetics and Evolution, 2025), highly consistent with current classification for the group, including the addition of the family Ixoxolidae to Caelifera, an African endemic group separated from Acrididae.
CAELIFERA
(29:2,575/)12,603 spp. worldwide and (9:264/)846 in Brazil, numbers based on a compilation of the data below analyzed by group, including 5 caeliferid famlies in New World unknown in Brazil.
ACRIDIDEA
(2,548/)12,352 spp. worldwide (Catalogue of Life) in 26 families — 15 are not recorded in New World (12 from Africa, including Ixalidiidae/SEE; three of them up to Eurasia and New Guinea; two from India to New Guinea; and one restricted to Australasian region) and 11 in New World: 7 in Brazil (259/815 in country) — Acrididae (cosmopolitan to subcosmopolitan, 148/421 in Brazil — CTFB/Orthoptera), Eumastacidae (cosmopolitan to subcosmopolitan, 11/32 in Brazil — CTFB/Orthoptera), Ommexechidae (Neotropics, 5/12 in Brazil — CTFB/Orthoptera), Proscopiidae (Costa Rica to South America, 15/106 in Brazil — CTFB/Orthoptera), Pyrgomorphidae (cosmopolitan to subcosmopolitan, 3/3 in Brazil — CTFB/Orthoptera), Romaleidae (North to South America, 53/173 in Brazil — CTFB/Orthoptera), and Tetrigidae (subcosmopolitan, 24/68 in Brazil — CTFB/Orthoptera) — and four unknown in Brazil: Tanaoceridae (W USA to NW Mexico), Episactidae (Mexico to Costa Rica, Hispaniola, Madagascar and E Asia), Xyronotidae (endemic to Mexico), and Tristiridae (Peru to S Argentina).
TRIDACTYLIDEA
(27/)251 spp. worldwide (Catalogue of Life), subcosmopolitan, in three families (2:5/31 spp. in Brazil, CTFB/Orthoptera): Tridactylidae subcosmopolitan (3/19 in Brazil — CTFB/Orthoptera), Ripipterygidae in tropical America (2/12 in Brazil — CTFB/Orthoptera), and Cylindrachetidae (3/16) from Argentina, disjunct in New Guinea to Australia, unknown in Brazil.
ENSIFERA
(14:2,493/)17,141 spp. worldwide and (10:317/)1,009 in Brazil, numbers based on a compilation of the data below analyzed by group, including 4 tettigonid famlies unknown in Brazil.
GRYLLIDEA
(801/)6,272 spp. (Catalogue of Life) in seven families (Jasso-Martínez, JM et al, Molecular Phylogenetics and Evolution, 2025), all in Brazil (116/339, CTFB/Orthoptera): Gryllidae (19/44 in Brazil — CTFB/Orthoptera), Gryllotalpidae (4/12 in Brazil — CTFB/Orthoptera), Mogoplistidae (1/2 in Brazil — CTFB/Orthoptera), Myrmecophilidae (1/63, 1 in Brazil, Anti Wiki | Colavite et al., Zootaxa, 2025), Oecanthidae (25/78 in Brazil — CTFB/Orthoptera), Phalangopsidae (48/146 in Brazil — CTFB/Orthoptera), and Trigonidiidae (18/56 in Brazil — CTFB/Orthoptera).
TETTIGONIIDEA
(1,692/)10,869 spp. (Catalogue of Life) in 7 families (Jasso-Martínez, JM et al, Molecular Phylogenetics and Evolution, 2025), four do not occur in Brazil: Schizodactylidae (2/20, Turkiye to Indochina, and SW Africa), Prophalangopsidae (5/8, Cyphoderris-3 in NW America, Paracyphoderris-1 in Siberia, Aboilomimus-2 in China, Prophalangopsis-1 in Indian subcontinent, and Tarragoilus-1 in China), Rhaphidophoridae (96/922, Alaska to Guatemala, Eurasia to New Zealand, South Africa, Argentina and Chile), and Stenopelmatidae (7/71, two genera in New World, Ammopelmatus from SW USA and NW Mexico, and Stenopelmatus from America Central and Ecuador in New World, Wikipedia, also Old World).
Three families (201/670) occur in Brazil: Anostostomatidae (3/8 in Brazil — CTFB/Orthoptera), Gryllacrididae (8/22 in Brazil — CTFB/Orthoptera), and Tettigoniidae (190/640 in Brazil — CTFB/Orthoptera).
NOTES
The most massive insects are wētā from New Zealand (6, Deinacrida, Anostostomatidae), with one mention of a individual of D. heteracantha White, 1842 specimen from extreme northern country weighing 71g (Williams, Book of Insect Records, 2005).
■ endemic families in New World: Xyronotidae (Caelifera, 2/4, Mexico).
6 ORDER PHASMIDA
(551/)3,595 spp. worldwide (Catalogue of Life) in 18 families (Wikipedia plus Tinematidae), 900 in New World and (5:69/)232 in Brazil (CTFB/Phasmatodea) among five families: Diapheromeridae, Heteronemiidae, Pseudophasmatidae, Prisopodidae, and Phasmatidae. Agathemeridae (8) occurs only in Argentina, Bolivia and Chile (Vera et al., Zoological Journal of the Linnean Society , 2012), and Tinematidae (21) occurs from Oregon to Baja California in NW Mexico (Wikipedia). All remaining families are invalid or not recorded in New World.
The longest known insect, based on leg length, is a species not described in Phasmatidae from China, from captivity, which reached 64cm (Wikipedia).
7 ORDER EMBIOPTERA
(14:89/)453 spp. worldwide (Catalogue of Life | EMBIOPTERA SPECIES FILES), (5:26/)58 in Brazil (CTFB/Embioptera: Anisembiidae, Archembiidae, Clothodidae, Oligotomidae and Teratembiidae) and (4:11/)58 in Mexico (Rafael et al., BOOK, 2024). Eight families are not recorded from in New World. Andesembiidae (2/8, Colombia to Peru) is the only New World family that is not recorded in from Brazil.
8 ORDER NOTOPTERA
(23/)54 spp. in two extremely distinct families, Mantophasmatoidae and Grylloblattodae, one from arid zones of subtropical Africa and the other from cold montane regions of the Northern Hemisphere.
GRYLLOBLATTIDAE
(5/)33 spp. (Schoville & Graening, Zootaxa, 2013 | Wipfler, B. et al., Journal of Insect Biodiversity, 2014 | Foottit & Adler, Insect Biodiversity, 2018), in Galloisiana (12, NW China, North and South Korea, SE Russia, and Japan), Grylloblattina (2, Primorsky Region of far‐eastern Russia), Grylloblattella (3, Altai and Khakassia republics in Russia, and N Xinjiamg regin of China), Namkungia (2, northern South Korea) and Grylloblatta (14, California, Oregon, Washington, Idaho, Montana, Alberta and British Columbia).
MANTHOPHASMATIDAE
(18/)21 spp. (Benjamin Wipfler et al., ZooKeys, 2017 | Wikipedia) in four subfamilies: Tanzaniophasmatinae (1/1, Tanzania, Malawi, Mozambique), Tyrannophasmatini (2/2, Nambia, one sp. up South Africa), Mantophasmatini (3/6, endemic to Namibia) and Austrophasmatini (9/12, South Africa, one sp. up to Namibia).
9 ORDER PLECOPTERA (PLECOPTERA SPECIES FILES)
(17:309/)3,788 spp. worldwide (Zhi-Qiang Zhang, Zootaxa, 2013 | Zootaxa, 2013 | Plecoptera Species File) in two suborders, both with a single family in Brazil (2:10/205): Antarctoperlaria with Gripopterygidae (4/63, CTFB/Plecoptera) and Arctoperlaria with Perlidae (6/142, CTFB/Plecoptera). Additonal data of Plecoptera in Brazil in Duarte & Lecci (Revista Brasileira de Entomologia, 2023). (7:12/)55 spp. in Mexico (Biodiversidad de Morelos | Sargent et al, The Southwestern Naturalist, 1991 | Stark & Kondratieff, MWNAN, 2004), mainly Perlidae (1/30), also the unbrazilian Capniidae (3/8), Leuctridae (1/1), Nemouridae (2/9), Chloroperlidae (2/2), Perlodidae (2/4) and Pteronarcyidae (1/1). Brazilian families in bold. Several informations in Eichert, A et al (Insect Systematics and Diversity, 2025).
ANTARCTOPERLARIA/EUSTHENIOIDEA
Diamphipnoidae (Argentina, Chile, Paraguay, Uruguay, SEE) and Eustheniidae (Argentina, Chile, Paraguay, Uruguay, Australia, New Zealand, SEE).
ANTARCTOPERLARIA/LEPTOPERLOIDEA
Austroperlidae (Argentina, Chile, Australia, New Zealand, SEE) and Gripopterygidae (South America except Venezuela, Australia to New Zealand, SEE).
ARCTOPERLARIA/SCOPURIDAE
Scopuridae (Korea and Japan, SEE).
ARCTOPERLARIA/EUHOLOGNATHA
Capniidae (300, Alaska to Mexico, Arctic to Algeria, India, also Java, SEE), Leuctridae (390, Alaska to Mexico, Arctic to Algeria, India and Indonesia, SEE), Nemouridae (Arctic to Mexico, over Eurasia to Australia, northern Africa, SEE), Notonemouridae (Argentina, Chile, Southern Africa, Madagascar, Australia to New Zealand, SEE) and Taeniopterygidae (110, Alaska to USA, Arctic to Algeria and India, SEE).
ARCTOPERLARIA/SYSTELLOGNATHA
Chloroperlidae (180, Arctic to NW Mexico, Marocco, Syris, Pakistan, Thailand, also in Java, SEE), Kathroperlidae (Alaska to California, Korea, SEE), Perlidae (400, cosmopolitan, SEE), Perlodidae (350, Artic to NW Mexico, Algeria, Syria, India and Thailand, SEE), Peltoperlidae (68, Canada to USA, Kazakhstan, India, NE Russia to Indonesia, SEE), Pteronarcyidae (12, Arctic to Mexico, Central Asia, Siberia to Korea, SEE) and Styloperlidae (10, China, Vietnan, Taiwan, SEE).
NOTES
Capnia lacustra Jewett, 1965 from Lago Tahoe (USA) and Baikaloperla (Lake Baikal), both Capniidae, are the only fully aquatic life cycle insects (Planeta Invertebrados).
10 ORDER DERMAPTERA (DERMAPTERA HOME PAGE)
(28:282/)2,188 spp. worldwide (Catalogue of Life), 303 in South America, (6:43/)147 in Brazil (CTFB | Earwings Online). All New World families occur in Brazil, which holds the largest diversity of Dermaptera in New World and the fouth worldwide after India (239), China (229) and Indonesia (204), by Earwings Online. (5:27/)51 spp. in Mexico (Insecta/MX).
11 ORDER ZORAPTERA
(9/)37 spp. in two families (Kočárek et al., MDPI, 2021). Brazil has the largest diversity of genera worldwide (2:4/6).
ZOROTYPIDAE
Three genera, Zorotypus (Kenya, Guinea, Ghana, and Ivory Coast, Madagascar, Mauritius and Brazil), Usazoros (endemic to USA) and Spermozorus (China, Malaysia, Indonesia).
SPIRALIZORIDAE
Six genera, Latinozoros (Panama, Costa Rica, Venezuela, República Dominicana, T.Tobago, French Guiana, Brazil, SEE), Spiralizoros (China, Vietnam, Malaysia, Indonesia, Sri Lanka, Philippines), Centrozoros (U.S.A, Jamaica, Guatemala, Costa Rica, Panama, Mexico/1-SEE, Colombia, Brazil, Ecuador, Peru), Brazilozoros (Brazil, Peru, Guyana, Ecuador), Cordezoros (Fiji) and Scapulizoros (Papua New Guinea).
12 ORDER MANTODEA
(30:457/)2,554 spp. worldwide (Catalogue of Life), (15:71/)229 in Brazil (CTFB/Insecta).
13 ORDER BLATTODEA
(17:815/)7,489 spp. worldwide in six clades (SEE), five clades (and 8 families) of cockroaches sucessively sisters of remaining groups. Among cockroaches, China and Australia have the largest diversities of families in World, six each. Such as cockroaches, curiously, China and Australia have the largest diversities of families in World, five each.
Among all Insecta groups, cockroach families show one of the most unstable classifications, with several sources adopting their own arrangements. For non-Isoptera Blattodea taxa, we follow the general classification of Beccaloni & Eggleton (Zootaxa, 2011), and the numerical data of Rafael et al. (Insetos do Brasil, 2024).
(9:220/)1,121 spp. in Brazil, numbers based on a compilation of the data below analyzed by group.
SUPERFAMILY CORYDIOIDEA
NOCTICOLIDAE ‣ (9/)32 spp. worldwide, from Africa, Asia and Australia, MAP, unknown in Brazil.
CORYDIIDAE ‣ (39/)215 spp. worldwide, (11/)19 in Brazil, genera based on CTFB/Insecta.
BLABEROIDEA
ECTOBIIDAE ‣ (223/)2,381 spp. worldwide, (69/)488 in Brazil, genera based on CTFB/Insecta.
BLABERIDAE ‣ (165/)1,198 spp. worldwide, (48/)238 in Brazil, genera based on CTFB/Insecta. Luminescent insects includes only members of Coleoptera and Diptera, thus the evidence for genuine bioluminescence in Lucihormetica cockroaches (Blaberidae) from Ecuador is anecdotal and inconclusive, though there is evidence for autofluorescence (Wikipedia).
BLATTODEA/BLATOIDAE
BLATTIDAE ‣ (41/)594 spp. worldwide, (4/)17 in Brazil, Polyzosteriinae composed by native species, and Blattinae includes only six exotic species, in Blatta, Periplaneta and Neostylopyga.
LAMPROBLATTIDAE ‣ (3/)10 spp. worldwide, (2/)4 in Brazil, in Lamproglandifera (1, Brazil and French Guiana) and Lamproblatta (America Central to South America, 3 in country).
TRYONICIDAE ‣ (10/)47 spp. worldwide, unknown in Brazil.
BLATTODEA/CRYPTOCERCOIDEA
CRYPTOCERCIDAE ‣ (1/)12 spp. worldwide, unknown in Brazil.
TERMITOIDEA
(9:324/)3,000 spp. worldwide (Termites Database), (4:85/)349 in Brazil (CTFB/Isoptera | CTFB for genera), in Serritermitidae (2/2, only in Brazil, Guyana and French Guiana), Kalotermitidae (28), Termitidae (305) and Rhinotermitidae (5/14).
5 families does not occur in Brazil: Hodotermitidae (3/18, Africa to S India), Mastotermitidae (1/1, Australia), Archotermopsidae (3/11, one genus in North America north of Mexico and two in E Asia), Stolotermitidae (2/10, South Africa, Australia, New Zealand, Porotermes quadricollis Rambur, 1842 in Argentina and Chile) and Stylotermitidae (1/34, Bangladesh, China, India, and Malaysia).
Hodotermitidae, Archotermopsidae, Stolotermitidae and Serritermitidae are fully disjunct range families. South America has (5:75/)422 spp. (Constantino, Encyclopedia of Entomology, 2008). In New World, Brazil, Guyana, French Guiana, Argentina and Chile has the largest family-diversities, with 4 each.
14 ORDER PSOCODEA
The insect order Psocodea includes the two groups historically recognized as the orders Psocoptera (free-living) and Phthiraptera (ectoparasites), which were treated as separate orders over time. However, phylogenetic studies based on morphological and molecular data have confirmed that Psocoptera and Phthiraptera together constitute a natural group. Some Troctomorpha, such as Liposcelis (which are similar to lice in morphology), are often found in birds' nests, and it is possible that a similar behavior in the ancestors of lice is at the origin of the parasitism seen today (Wikipedia).
(88:926/)12,028 spp. worldwide (Catalogue of Life), (47:255/)1,058 in Brazil (CTFB/Insecta).
All suborders and infraorders of New World in this order occur in Brazil. Mexico has (37:105/)755 spp. in former Psocoptera (Garcia-Aldrete, Revista Mexicana de Biodiversidad, vol. 85, 2014) and (16:123/)443 in former Phthiraptera (Torres & Santana, Revista Mexicana de Biodiversidad, 2021), totaling (53:228/)1,199 spp.
In former Phthiraptera, 7 families do not occur in Brazil, six in Anoplura, and Haematomyzidae (3, from elephants and warthogs, Wikipedia). Echinophthiriidae (5/13, unknown in Brazil) are parasites of seals and the river otter, and are the only insects that infest aquatic hosts (Wikipedia). Pedicinidae (1/14) infects only Old World monkeys (Wikipedia).
15 ORDER TYSANOPTERA (WIKITRIPS)
(10:820/)6,588 spp. worldwide (Catalogue of Life), (6:166/)645 in Brazil (CTFB/Insecta).
Families unknown in Brazil are 4: Adiheterothripidae, Melanthripidae (Cranothrips in Australia and South Africa, Dorythrips in Australia and W South America, Ankothrips in USA, Europe and South Africa, and Melanthrips in Europe, Africa, India and North America), Fauriellidae (4/5, one in California, two from S Africa, and two from S Europe) and Stenurothripidae (13 /25, two in W North America, remaining in Old World). 8 families occur in USA.
16 ORDER HEMIPTERA
(200:16,076/)106,014 spp., numbers based on a compilation of the data below analyzed by order, in four lineages (Wikipedia), one unknown in Brazil. (117:2,330/)9,601 spp. in Brazil (CTFB/Insecta).
AUCHENORRHYNCHA
(35:6,497/)46,096 spp. worldwide (Catalogue of Life), (24:878/)3,747 in Brazil (CTFB/Insecta, manual counting). Auchenorrhyncha includes Cicadomorpha and Fulgoromorpha; for a complete phylogeny of Fulgoromorpha, see Bucher et al. (Molecular Phylogenetics and Evolution, 2023).
COLEORRHYNCHA
(20/)40 spp. in two families, Peloridiidae and Permoridiidae, known from Argentina, Chile, New Zealand, New Caledonia, and E Australia, living in the wet moss of temperate and subantarctic rainforests (Catalogue of Life | Burckhardt, Larochelle & Lariviere, BOOK, 2011).
HETEROPTERA
(98:6,781)40,329 spp. worldwide (Catalogue of Life), (59:1,090/)4,811 in Brazil (CTFB/Insecta, manual counting).
Halobates (46, Gerridae, sea-skaters) includes the only true marine insects. Aside from some members of Collembola, Diptera, Coleoptera, and other Hemiptera that inhabit intertidal or coastal environments worldwide (M. Springer, BOOK, 2009), five species of Halobates live on the surface of the open ocean, approaching coastal areas only when storms wash them ashore (Wikipedia). These are the only insects known to be truly oceanic. Of these, four occur along the Pacific coast of the Americas (H. robustus Barber, 1925, endemic to the Galápagos Islands; H. sobrinus White, 1883, and H. splendens Witlaczil, 1886), one of which, H. micans Eschscholtz 1822, also occurs in the Atlantic and represents the only species of the group recorded from Brazil (N. M. Andersen & L. Cheng, Oceanography and Marine Biology, 2004 | MAP | Dias, JF. and Lopes, CL., Braz. J. Biol., 2009).
STERNORRHYNCHA
(65:2,778/)19,547 spp. worldwide (Catalogue of Life), (34:346/)949 in Brazil (CTFB/Insecta, manual counting).
■ endemic families in New World: Curaliidae (1/11, USA), Pityococcidae (2/5, USA).
17 ORDER HYMENOPTERA
(160:13,506/)146,440 spp. worldwide (Catalogue of Life). Almost all Hymenoptera are wasps in appearance except two lineages, family Formicidae and Apoidea clade (11 families, Wikipedia). (92:1,681/)11,364 in Brazil (CTFB/Insecta).
BEES
7 families, 5 in Brazil (Andrenidae, Apidae, Colletidae, Halictidae, Megachilidae), Melittidae (187, Africa and northern Hemisphere) and Stenotritidae (2/21, endemic to Australia), by Wikipedia (Bees) and Rafael (2012). Bombus has 250 spp. widely distributed in the World, but occurring mainly in the cool subtropical and temperate areas of the Nearctic and, especially, Palearctic regions. In South America, most of the species of Bombus are distributed along the Andes and in temperate regions, with only a few species recorded in the warm lowlands—the later, actually, are the only bumblebees to occur in such environments in the world. Only six spp. of the genus are generally referred to occur in Brazil (JE Santos Júnior, Plos One, 2015).
By Engel et al. (ZooKeys, 2023), Neotropical Meliponi includes (16/)475 spp., with 16 genera widely widespread from Mexico to Brazil (joined 449 spp.), sometimes up to Argentina, Uruguay or Bolivia, plus two only from Mexico to Panama (3), Ptilotrigona and Nogueirapis from Costa Rica to Brazil (12), Paratrigonoides (1) endemic to Colombia, Mourella and Schwarziana from Brazil and adjacent C & S South America (5), Duckeola (2) from Brazil, Ecuador, French Guiana, Colombia, and Friesella (1) and Trichotrigona (2) both endemic to S Brazil.
FORMICIDAE
Following the Ant Wiki/Diversity by Country, Brazil has the greatest diversity of genera in the West Hemisphere (117) and the 3rd in the world, behind only Indonesia (126) and China (122); 2rd largest diversity of endemic genera (10), behind only Australia (16); 2rd largest species diversity (1,610), behind only Australia (1,619); 4rd country with the most endemic species, with 508, behind only Australia (1,406), Madagascar (743) and New Guinea (530).
SOCIAL WASPS
Among wasp families, only Vespidae contains eusocial species, exclusively in Vespinae and Polistinae (Wikipedia). In Brazil there are (20/)381 spp., 2/3 in Mischocyttarus (144), Polistes (43) and Polybia (51) genera (Somavilla et al., Springer, 2020). All South American social wasps are Polistinae (Rafael, 2024), subfamily of which Brazil has the largest diversity worldwide, with 21 of 25 genera - exceptions are the four genera of Ropalidiini, subtribe from Afrotropical, Indomalayan and Australasian biogeographical regions (PCS Barroso, A Somavilla, R Boldrini, Sociobiology, 2017).
Vespinae includes 4 genera (Wikipedia), mainly in Old World. In New World, natively, occur only Vespula (14 in region, USA/Canada-12, Mexico-6 and Guatemala-1) and Dolichovespula (6) from Canada and USA (Kimsey & Carpenter, Journal of Hymenoptera Research, 2012 | Dvorak, Entomological Problems, 2006).
RECORDS
Two Mymaridae wasps are incredible minuscule: Dicopomorpha echmepterygis Mockford, 1997 is the smallest known insect, with a body length averaging 186 μm (smaller than certain species of Paramecium and amoeba, which are single-celled organisms), know as an idiobiont parasitoid of the eggs of a lepidopsocid barklouse, Echmepteryx hageni, in Illinois, USA (Wikipedia); and Kikiki huna Huber & Beardsley, 2000, known from Hawaii, Costa Rica, Argentina (SEE), S India and T.Tobago; at 0.15 mm, it is the smallest flying insect known as of 2019 (Wikipedia).
Xylocopini (carpenter bees) produced the largest insect eggs in absolute terms (Vicidomini, POST, 2005).
Dinoponera may have a body length exceeding 3.5cm, making them the genus with the largest known ant workers; they are exclusively South American, with their center of diversity in Brazil, the only country where all described species can be found (Dias & Lattke, EJT, 2021).
18 ORDER STREPSIPTERA
(12:53/)648 spp. worldwide (Catalogue of Life). Brazil has (13/)33 spp., in 7 families (CTFB/Insecta): Bahiaxenidae (1/1, endemic to Brazil), Corioxenidae (1/2), Elenchidae (1/1), Halictophagidae (2/6), Myrmecolacidae (3/3), Stylopidae (2/3), Xenidae (3/17). All families of New World occur in Brazil (Tolweb) except Bohartillidae (3, Honduras and Hispaniola, Wikipedia). (5:)16 in Mexico (SEE | SEE).
■ endemic families in New World: Bahiaxenidae (1/1, Brazil).
19 ORDER COLEOPTERA
(211:39,258/)372,186 spp. worldwide (Catalogue of Life, includes especies provisionally accepted).
(118:5,012/)36,258 spp. in Brazil (CTFB/Insecta), including representatives of all extant suborders and superfamilies, being the richest on the planet, concentrating 1/11 of the world species diversity. The most diverse family in numbers of genera is Cerambycidae (1,056 genera), while in number of species it is Chrysomelidae (6,079 spp.). Conotrachelus Dejean, 1835 (Curculionidae) is the most species-rich genus, with 570 spp.
Badmaateridae is a recently described monotypic family endemic to S Chile and is still absent from many databases (Grebenikov, VV et al, Systematic Entomology, 2026).
The three basal suborders Archstemata, Myxophaga and Adephaga has 20 families, 11 in Brazil. Italy and Russia one endemic each, two scattered in Old World, Sphaeriusidae (22) is highly centered in Asia (Zu-long Liang & Fenglong Jia, ZooKeys, 2018) with a single described species in Argentina (Beuteu & Raffaini, Koleopt. Rdsch., 2003). Lepiceridae is found from Mexico to Venezuela and Ecuador (Wikipedia). Amphizoidae (5) has three species in W North America and two in eastern palearctic. Meruidae (1) contains only Meru phyllisae Spangler & Steiner 2005 from aquatic places in Venezuela. Trachypachidae (6) has four species of Trachypachus in northern Eurasia and N North America, and two species of Systolosoma in Chile.
FIREFLIES
Luminescent insects includes only members of Coleoptera and Diptera. The presence of bioluminescence in Coleoptera is exclusive in the six families: Elateridae (147), Phengodidae (34/263, all luminescent/SEE, 53 in Brazil), Rhagophthalmidae (Old World) and Lampyridae (2,000 spp., all luminescent/SEE, 362 in Brazil), Omalisidae (factum dubia) and Staphylinidae (in two undescribed species of Xantholius exclusive from Brazil, Rosa, Revista Brasileira de Entomologia, 2010).
147 spp. of Elateridae are able to emit light, in four lineages: Agrypninae (Pyrophorini, 30/159, SEE), Thylacosterninae (Balgus schnusei Heller from Bolivia, Brazil, Peru and French Guiana, SEE), Sinopyrophorinae (Sinopyrophorus schimmeli Bi & Li, 2019, China, SEE) and Campyloxeninae (Campyloxenus pyrothorax Fairmaire, S Chile and Argentina, SEE).
In these numbers, there are approx. 2,410 spp. of fireflies in the world, with Brazil having the largest diversity of luminescent beetles in the world, about 417-557 described species, corresponding about 23% of described species in the world.
NOTES
One high remarkable beetle from Brazil (and Bolivia) is the Pharaxonotha cerradensis Skelley & Segalla 2019 (Erotylidae), highly associated with Zamia boliviana (Brongniart) A. DC. (Cycadales: Zamiaceae), by Skelley & Segalla (Zootaxa, 2019). Xenomorphon baranowskii Ferreira & Barbosa & Bocakova & Solodovnikov, 2023 (Lycidae) is the unique completely anelytrous and wingless adult male beetle, known only from S Mexico (Ferreira et al., Zoological Journal of the Linnean Society, 2023). Onychocerus albitarsis Pascoe, 1859 (Cerambycidae) is a relatively rare species of beetle from the Amazon in N Brazil, Bolivia, Paraguay and S Peru, and Atlantic Forest regions in E Brazil, remarkable for be the only known beetle that has a venomous sting (as opposed to spraying toxins like bombardier beetles or secreting toxins from the body like blister beetles) and the only known arthropod that stings with its antennae (Wikipedia).
RECORDS
Titanus giganteus L., 1771 (Cerambycidae) is the largest Coleoptera worldwide, measurng up to 16,3cm body lenght, known widely in northern South America (Wikipedia). Scydosella musawasensis Hall, 1999 (Ptiliidae) is regarded as the smallest free-living insect, as well as the smallest beetle (0.300 mm in length), and is known disjunctly from Nicaragua and C Colombia (Wikipedia).
■ endemic families in New World: Diphyllostomatidae (1/3, USA), Jurasaidae (2/5, Brazil), Meruidae (1/1, Venezuela).
20 ORDER NEUROPTERA
(16:1,044/)5,997 spp. worldwide (Catalogue of Life), (10:82/)442 in Brazil (211 endemic, CTFB/Insecta ). Mexico has (10:)349 spp. in this order (Contreras-Ramos, Revista Mexicana de Biodiversidad, vol. 85, 2014).
21 ORDER MEGALOPTERA
(2:54/)399 spp. worldwide (Catalogue of Life). (4/)23 spp. in Brazil in both families (CTFB/Megaloptera). (2:5/)13 spp. in Mexico (Contreras-Ramos & Rosas, Rev. Mex. Biodiv., 2014).
CORYDALIDAE
(25/)315 spp. worldwide, 8 genera in Neotropics: Chloronia (18, Neartic and Neotropical, 5 in Brazil), Corydalus (39, Asia and New World, 14 in Brazil), Platyneuromus (3, Mexico to America Central), Archichauliodes (21, Chile, Australia, New Zealand), Neohermes (6, USA to Mexico), Nothochauliodes (1, Chile), Protochauliodes (13, USA, Australia, Chile), and Puri (1, endemic to Brazil). Two genera in Chile, 3 in Brazil and USA each, and 4 in Mexico.
SYALLIDAE
(9/)85 spp. worldwide, two genera in Neotropics: Caribesialis (1, Cuba) and Ilyobus (10, Mexico to South America, 3 in Brazil).
22 ORDER RAPHIDIOPTERA
(43/)260 spp. in two families from northern Hemisphere, Raphidiidae (36/213) and Inocelliidae (7/47) worldwide (Catalogue of Life), both exclusive from northern Hemisphere. In New World, they are found west of the Rocky Mountains, and range from SW Canada to the Mexican-Guatemalan border, which is the farthest south they have been found in the western hemisphere (Wikipedia), being 30 spp. in region (Aspock, Acta Zool. Fennica, 1998). (4/)14 spp. in Mexico in both families (Aguna, Alena, Indianoinocellia, and Negha, Contreras-Ramos & Rosas, Revista Mexicana de Biodiversidad, 2014).
23 ORDER TRICHOPTERA
(55:1,017/)13,680 spp. worldwide (Catalogue of Life), (16:73/)983 in Brazil (CTFB/Insecta).
Of the 7 major Wallacean biogeographic regions, the Indo-Malayan region has the highest number of named species and genera, with 5,853 and 207, respectively. It is followed by the Neotropical region with 3,309 named species in 171 genera. The Australasian region has the highest proportion of endemicity, with 6 endemic families and 125 endemic genera. Tropical or mountainous areas with significant precipitation levels are known to have high species endemism and are important areas to explore for continued species discovery (Frandsen et al., Insect Systematics and Diversity, 2025).
24 ORDER LEPIDOPTERA
(163:21,109)176,717 spp. worldwide (Catalogue of Life), (77:2,983/)14,234 in Brazil (CTFB/Insecta). Mexico has 14,507 spp. in this order (Llorente-Bousquets et al., Rev. Mex. de Biodiv., vol. 85, 2014). 4 groups.
AGATHIPHAGIDAE
(1/)2 spp. found along the NE Queensland (Australia), Fiji to Vanuatu and the Solomon Islands (Wikipedia).
HETEROBATHMIINA
A single genus, Heterobathmia (10), from S Argentina and Chile (Wikipedia).
ZEUGLOPTERA
(20/)180 spp. at Micropterigidae (Grehan, ZooNova, 2025), found in all continents, but only five genera in New World: Magnijuxta and Sporaphaga endemic to Costa Rica, Squamicornia in Costa Rica and Ecuador, Hypomartyria in Osorno region in Chile and Epimartyria (3) for British Columbia to California, and Quebec to Georgia in North America.
GLOSSATA
Remaining all members of order.
NOTES
Very aberrant lifestyles appear so often in moths and butterflies, such as females, apterus or sessile, ephemeral adults that do not feed, predatory larvae and some social ones. The adults of a primitive family (Micropterigidae, unknown in Brazil), retains chewing jaws, and caterpillars in some genera of Pyralidae and Arctiidae are aquatic. Larvae of at least two moth species, one of Arctiidae and the other of Saturniidae, are of medical interest, as they cause 'lepidopterism' or allergies that lead to hypersensitivity and, not infrequently, to death; many other larvae from these families and also from Limacodidae and Megalopygidae (fire caterpillars or caterpillars) cause very painful burns (FAPESP, link down). Hapialidae includes one genus of bizarre giant moth, Trichophassus giganteus Herrich-Schäffer, 1853, endemic to Brazil (X). Some Andean species of Nymphalidae overflying rocky slopes and resting directly on snow-covered surfaces, which is an exceptionally unusual behaviour among butterflies (Pyrcz, T. et al., ZJLS, 2024). Bombycidae has a Gondwanan origin (Lin, R.J. et al., Molecular Phylogenetics and Evolution, 2024).
MONARCH MIGRATION
Danaus plexippus L., 1758 (monarch butterfly, Nymphalidae) executes the most incredible insect migration known, flying, in generations, more than 3,000 km from western North America to a mountainous region between the Mexican states of Jalisco and Mexico. However, it should be noted that its two congeneric species, D. erippus Cramer, 1775 from South America and D. cleophile Godart, 1819 from the Caribbean, do not migrate. Not even all populations of D. plexippus migrate, and some more isolated groups migrate in a much more discreet way to other areas of North America (Wikipedia).
RECORDS
Thysania agrippina Cramer, 1776 (Erebidae), native from S Texas to Uruguay, has a measure of wingspan of 289mm for a Brazilian specimen (IMAGE), record for any insect (Kons, POST, 2005); a note about the larva of this insect is published on Facebook (POST).
Guinness World Records classified Lonomia obliqua Walker, 1855, a Saturniidae from S & SE Brazil, NE Argentina, Paraguay and Uruguay, as the most venomous caterpillar in the world. In a 2021 study, caterpillar accidents in Brazil attributed to Lonomia obliqua between 2007 and 2017 amounted to 42,264 recorded cases. Of the 248 cases considered severe, five resulted in death. While such incidents are not common, a Sao Paulo-based vaccine company has begun producing antivenom (Wikipedia).
25 ORDER DIPTERA
(210:11,345/)171,712 spp. worldwide (Catalogue of Life), (106:2,039/)12,261 in Brazil (CTFB/Insecta ). For Neotropical Diptera and many notes of presence of families in Neotropics, see Amorim (Chapter, 2009).
NOTES
One of the most fantastic diversity of diptera in the world is the Drosophillidae from Hawaii. As of 2022, 689 spp. have been described, including 273 spp. in the genus Scaptomyza, of which 148 are endemic to the Hawaiian archipelago, and 416 Hawaiian endemic species in the genus Drosophila (Wikipedia). Mexico includes 244 spp. of Culicidae (Ortega-Moralez, Journal of Vector Ecology
, 2023).
Luminescent insects includes only members of Coleoptera and Diptera. In Diptera, luminescence is found in Keroplatidae in four genera worldwide: Australasian Arachnocampa, Euroasiatic Keroplatus, North American Orfelia fultonii Fisher, 1940, and Brazilian Neoceroplatus betaryiensis Falaschi, Johnson & Stevani, 2019, known only S São Paulo state, unique in Neotropics (Photobiology | Falaschi, Johnson & Stevani, Nature, 2019). A world checklist of all fly Keroplatidae is available in Evenhuis (Bishop Museum Press, 2006); Neotropics is the poor region in diversity: only 92 out of a total of 952.
RECORDS
The most mortal family of animals, Culicidae, has (42/)3,492 spp. among 12 tribes. 24 genera occur in Neotropics (Balian, 2008), with 1,069 spp. Chagasia, Galindomyia, Isostomyia, Johnbelkinia, Limatus, Onirion, Sabethes, Shannoniana and Trichoprosopon are endemic to Neotropics. Tribe Ficalbiini and Hodgesiini are the unique not recorded in Neotropics. Only one Aedeomyiini occurs in New World (Aedeomyia squamipennis Lynch Arribálzaga, 1878, Nascimento Pereira et al., CheckList, 2017).
The smallest of all Diptera worldwide is Megapropodiphora arnoldi Brown, 2018 (Phoridae), known from a single specimen from a site near Manaus, N Brazil, is only 0.395 mm in body length, slightly smaller than the currently recognised smallest fly, Euryplatea nanaknihali Brown, 2012 from Thailand (Brown, Biodiversity Data Journal, 2018). On the other hand, the largest of all Diptera is Gauromydas heros Perty, 1833, mensuring up to 70mm body, known only from E & C Brazil up to E Paraguay (Calhau et al., Zootaxa, 2015).
Belgica antarctica Jacobs, 1900 (Chironomidae) is a species of flightless midge, endemic to the continent of Antarctica. At 2–6 mm long, it is the largest purely terrestrial animal native to the continent and has the smallest known insect genome as of 2014, with only 99 million base pairs of nucleotides and about 13500 genes. It is also the only insect that can survive year-round in Antarctica (Wikipedia).
■ endemic families in New World: Evoocoidae (1/1, Chile).
26 ORDER SIPHONAPTERA
(22:317/)2,223 spp. worldwide (Catalogue of Life). In Brazil occur (8:19/)63 spp. (Rafael et al., BOOK, 2024 | CTFB/Insecta ), in Ceratophyllidae (1/1), Ctenophthalmidae (1/5), Ischnopsyllidae (5/5), Leptopsyllidae (1/1), Pulicidae (3/5), Rhopalopsyllidae (5/35), Stephanocircidae (1/1) and Tungidae (2/10).
Pygiopsyllidae (W Amazonia) and Malacopsyllidae (known in Argentina) occur in neighboring countries and will probably be registered in Brazil. Mexico (8:51/172, Acosta-Gutiérrez, Revista Mexicana de Biodiversidad, vol. 85, 2014) and Argentina (108) has more species than Brazil, and Neotropics has (52:)280 spp. in 52 genera. Three genera (Rothschildopsylla, Hechtiella, Neotropsylla) and 17 spp. are Brazilian endemic (Fapesp). All Mexican families occur in Brazil except Hystrichopsyllidae, and Brazilian Stephanocircidae does not occurs in Mexico.
Mexican diversity ✕ Brazilian diversity: Ceratophyllidae (MX 18/69 ✕ 1/1 BR), Ctenophthalmidae (MX 12/45 ✕ 1/5 BR), Ischnopsyllidae (MX 5/10 ✕ 5/5 BR), Leptopsyllidae (MX 2/3 ✕ 1/1 BR), Pulicidae (MX 8/20 ✕ 3/5 BR), Rhopalopsyllidae (MX 2/8 ✕ 5/35), Hystrichopsyllidae (MX 2/8 ✕ 0 BR) and Tungidae (MX 2/2 ✕ 1/10 BR).
27 ORDER MECOPTERA
(10:68/)892 recent spp. worldwide (Catalogue of Life), (2:5/)26 in Brazil (CTFB), in Bittacidae (4/25) and Meropeidae (1/1).
In Neotropics occur four families and 67 spp.: Meropeidae (2/3, Merope in USA/Canada, Austromerope in Australia and Brazil, one each, Wikipedia), Eomeropidae (1/1, endemic to Chile), Nannochoristidae (3, Argentina and Chile), and Bittacidae (16 genera: 5 endemic to Australia, Anomalobittacus from South Africa, three from SW USA to Mexico, Kalobittacus in America Central, 4 from Panama to Brazil — all in Brazil, Neobittacus endemic, one endemic to Chile, and Bittacus worldwide). Mexico has (2:5/)47 spp. in this order (Contreras-Ramos, Revista Mexicana de Biodiversidad, vol. 85, 2014).
■ endemic families in New World: Eomeropidae (1/1, Chile).
▉ LAST UPDATE OF ARTHROPODA IN JANUARY/FEBRUARY 2026
DEUTEROSTOMIA
30. ECHINODERMATA ‣ five living class of sea animals (none in freshwater) and (239:1,472/)7,894 accepted living spp. worldwide, all classes in Brazil (97:210/356). Taxonomy follows Wikipedia. Only 7 of 38 orders worldwide do not occur in Brazil (Cyrtocrinida, Hyocrinida, Pedinoida, Echinoida, Phymosomatoida, Holectypoida and Peripodida). All data presented below follow Catalogue of Life/Echinodermata at the global level and CTFB/Echinodermata for Brazil, with occasional additions highlighted in the text.
For all 61 spp. of Echinodermata from Santa Catarina state (Brazil), see Slivak, N.N. et al. (Pap. Avulsos Zool., 2022). Currently, 643 spp. are known from Mexico, in Ophiuroidea (197), Asteroidea (185), Echinoidea (119), Holothuroidea (113) and Crinoidea (29), by Solís-Marín et al. (Echinoderm Research and Diversity in America Latina, 2012).
Some national checklist worldwide includes Crinoidea from Cuba (10:20/33 spp., Rodríguez-Barreras et al., Caribbean Journal of Science, 2013) and Echinoderms from SW Portugal (60 spp., Jesus & Fonseca, Boletín del Instituto Español de Oceanografía, 1999). 356 spp. have been recorded in Cuba of these, the class Crinoidea is represented by 34 species, Asteroidea by 76 species, Ophiuroidea by 162, Echinoidea by 63, and Holothuroidea by 51 (Valle-García, V et al., ERDLA, 2012).
CRINOZOA/CRINOIDEA
(54:212/)788 spp. worldwide, (8:16/)21 in Brazil (CTFB/Echinodermata | Promachocrinus kerguelensis Carpenter, 1879 new genus and species for Brazil), in two of four Crinoidea orders — exceptions are Cyrtocrinida-8 (/Worms) and Hyocrinida.
Comactinia auxiliadorae Souza, Martins, Fukuda & Souto, 2025 (Comatulidae) represents the first Crinoidea from the Brazilian coast described in the last 100 years and the first crinoid species from Brazil described by Brazilian researchers (Souza, TH et al., Zootaxa, 2025).
ECHINOZOA/HOLOTHUROIDEA
(35:283/)1,840 spp. worldwide and (16:42/)72 in Brazil, in all seven orders within this class. A. K. Miller et al. (Molecular Phylogenetics and Evolution, 2017) proposed a new classification based on genetic aspects, where some of the 5 traditional groups were dissolved. This file brings a beautiful art about the diversity of Holothuroidea. For data of some species in Brazil, see Moura et al. (Zootaxa, 2015).
An abundant population of elpidiid holothurians collected at 10,908 m in the Mariana Trench represents the deepest known deuterostome taxon (Gallo, N.D. et al, Oceanographic Research Papers, 2015).
ECHINOZOA/ECHINOIDEA
(65:294/)1,032 spp. worldwide in 13 orders, four of these not recorded in Brazil (Pedinoida, Echinoida, Phymosomatoida and Holectypoida). (23:42/)55 spp. in Brazil (CTFB | Lytechinus variegatus (Lamarck, 1816), in Manso, CLC, Biota Neotropica, 2008, first record of Temnopleuroida order in Brazil, uncited in CTFB in Feb 16, 2026).
ASTEROZOA/OPHIUROIDEA
(39:291/)2,202 spp. worldwide, (25:61/)138 in Brazil, in all six orders within this class.
ASTEROZOA/ASTEROIDEA
(46:392/)2,032 spp. worldwide, (25:49/)70 in Brazil in seven of eight orders. The unique not recorded in is Peripodida (4, Xyloplax, Wikipedia) from Bahamas, New Zealand and northern Pacific from Canada to Costa Rica (MDPI).
▉ LAST UPDATE OF ECHINODERMATA IN JANUARY/FEBRUARY 2026
31. HEMICHORDATA ‣ (7:27)149 spp. worldwide in two classes: Enteropneusta (4:22/113) and Pterobranchia (3:3/26). Tassia et al. (Plos One, 2016) lists all Hemichordata worldwide as of that date, in areas referenced in Spalding et al. (BioScience, 2007). (3:6/)11 spp. in Brazil.
All data presented below follow Catalogue of Life/Hemichordata at the global level, and CTFB/Hemichordata and Tassia et al. (Plos One, 2016) for Brazil, with occasional additions highlighted in the text.
ENTEROPNEUSTS
4 families and (24/)117 spp. worldwide, and (2:5/)10 in Brazil.
HARRIMANIIDAE ‣ (10/)41 spp. in Harrimania (4), Horstia (1), Mesoglossus (6), Meioglossus (1, possibly 7 undescribed, Defourneaux et al., PRE-PRINT, 2023), Ritteria (1), Protoglossus (4), Saccoglossus (19), Saxipendium (2), Stereobalanus (2) and Xenopleura (1). Only one sp. in Pacific Mexico, Saccoglossus pusillus Ritter, 1902 (Deland et al., Zootaxa, 2010). Unknown in Brazil.
TORQUARATORIDAE ‣ (5/)8 spp. (Catalogue of Life | Jabr. et al., Canadian Journal of Zoology, 2013), all in northern Hemisphere, in Artic Canada, N Atlantic, Russia Arctic and E & NE Pacific.
PTYCHODERIDAE ‣ (5/)48 spp. (Wikipedia) in Ptychodera, Balanoglossus and Glossobalanus. (3/)8 spp. in Brazil (Tassia et al., Plos One, 2016): Balanoglossus (5, CTFB/Hemichordata), Ptychodera bahamensis Sprengel, 1983, Glossobalanus minutus Kowalevsky, 1866 (collected only in Brazil, Italy and Portugal - Sealifebase) and G. crozieri van der Horst, 1924 (collected only in Brazil and Bermuda - Petersen & Ditadi, Marine Biology, 1971).
SPENGELIDAE ‣ (4/)20 spp. in Spengelia (6), Glandiceps (8), Willeyia (3) and Schizocardium (3), found exclusively in tropic and sub-tropic waters, with only six spp. found outside the latitudinal boundaries of the Tropic of Cancer and the Tropic of Capricorn, and none are known from wholly temperate regions. (2/)2 spp. in Brazil, Schizocardium brasiliense Spengel, 1893 and Willeyia loya Petersen, 1965 (Tassia et al., Plos One, 2016).
PTEROBRANCHIA
(3:3/)32 spp. in three monogeneric families (Catalogue of Life): Atubariidae (1/1), Cephalodiscidae (1/19) and Rhabdopleuridae (1/12). Here we consider a record of Cephalodiscus in Brazil, an unnamed species, unfortunately it was never identified, based on 16 individuals collected during prospecting between 1996 and 1997 and 10 collected in 2001-2002, in Rio Grande do Sul (Haimovici, REVIZEE, 2004; Haimovici, REVIZEE, 2008).
▉ LAST UPDATE OF HEMICHORDATA IN JANUARY/FEBRUARY 2026
32. CEPHALOCHORDATA ‣ (3/)31 spp. worldwide and (2/)3 in Brazil in a single family, Brachiostomatidae (Zhang et al., Frontier of Physiology, 2018), numbers based on a compilation of the data below analyzed by genus.
Although Catalogue of Life places Cephalochordata (and Tunicata) within Chordata, we here recognize them as independent phyla, following the rationale discussed in the introduction
Asymmetron
Asymmetron, taken here as a single species, A. lucayanum Andrews, 1893, is reported to be composed of 5 lineages (Subirana, L. et al., Plos One, 2020), three of which have already been nominally described: A. inferum Nishikawa, 2004 (Japan, Nishikawa, Zoological Science, 2004), A. lucayanum (Indo-West Pacific Clade), Red Sea Clade (A. rubrum Subirana, Farstey, Bertrand & Escriva, 2020, Plos One), Atlantic Clade (C, Caribbean Basin from Bermudas to Yucatan and Lesser Antilles) and West-Central Pacific (B).
The distribution for the genus accepted here is the combination of: GBIF/Asymmetron, Copepedia/Asymmetron (excluding Baltic records), Kon et al. (Marine Biology, 2006), (Canul-Quiñonez, CI et al., Check List, 2025) and J. E. Carvalho et al. (Int. J. Dev. Biol., 2017).
The status of Asymmetron in Brazil is ambiguous. Its occurrence in the country has never been recorded for adult individuals. Bjornberg (Boletim do Instituto Oceanográfico, 1954) made a historical record of Acrania larvae (two speciemens) in Fernando de Noronha, from a taxon that is sometimes considered valid as Amphioxides pelagicus (Günther) 1889 in some sources (Goldschmidt, Biological Bulletin, 1933| Tommasi, L.R. et al., Boletim Marítimo do Instituto Oceanográfico, 1972), while in others it is assigned to Asymmetron (Rodrigues, S.A., SBZ, 1987), which would validate the species presence in the country. Adopting the second option, we accept Asymmetron in Brazil, as supported by OBIS (SEE, 2025) and CTFB/Branchiostomatidae.
Branchiostoma
25 spp., widely distributed in the mid-low latitudes of the Atlantic Ocean, the Mediterranean, and the Pacific Ocean (Poss & Boschung, Israel Journal of Zoology, 1996). Two spp. in Brazil (CTFB/Branchiostomatidae) — however, Alves et al. (Tropical Oceanography, 2001) lists B. marambaiensis Silva, 1977, rejected here and in Catalogue of Life.
Epigonichthys
5 spp., from Pacific, northern Indian Ocean near Thailand, S Japan, Timor island in Indonesia, around Australia, N & C New Zealand, and Hawaii (Copepedia/Epigonichthys | Catalogue of Life), unknown in New World.
▉ LAST UPDATE OF CEPHALOCHORDATA IN JANUARY/FEBRUARY 2026
33. TUNICATA ‣ many references (such as Catalogue of Life/Tunicata and CTFB) treat Tunicata as part of the phylum Chordata, including three orders (Ascidiacea, Thaliacea, and Appendicularia). In this work, we adopt Tunicata as an independent phylum, with Chordata restricted to the clade Craniata. We also accept the synonymization of Thaliacea under Ascidiacea (Kocot K.M. et al., Molecular Phylogenetics and Evolution, 2018), thereby reducing Tunicata to just two classes. With these changes, we consider Tunicata a phylum comprising (39:241/)3,076 spp. worldwide and (23:71/)224 spp. in Brazil, numbers based on a compilation of the data below analyzed by order.
ASCIDIACEA
After the inclusion of Thaliacea in Ascidiacea, both with three orders, here we take six orders and 34 families in the class. (34:221/)3,006 spp. worldwide (Catalogue of Life/Thaliaceae | Catalogue of Life/Ascidiacea) and (20:61/)180 in Brazil (under Craniata, CTFB/Ascidiacea, CTFB/Thaliacea).
Records of the predatory tunicate Octacnemidae sp. observed nine times at 7,799 m in the Mariana Trench and once at 8,077 m in the Izu-Ogasawara Trench (NW Pacific Ocean) are the deepest worldwide from Tunicata (Jamieson, AJ et al., Marine Biology, 2023).
APPENDICULARIA
(5:21/)70 spp. worldwide (Catalogue of Life), (3:10/)44 in Brazil (CTFB/Appendicularia).
▉ LAST UPDATE OF TUNICATA IN JANUARY/FEBRUARY 2026
34. CRANIATA ‣ the classification of Craniata is subject to some controversy in the current literature. Some sources group Cephalochordata and Tunicata together with Craniata within a broader phylum called Chordata. In this work, however, we treat these three groups as distinct phyla. Fish and reptiles are often addressed under Craniata in a broad sense. Here, however, we focus strictly on monophyletic units. Following Eschmeyer's Catalog of Fishes (SEE), 'fish' is used as a general term for eight distinct and basal classes within the phylum (the nomenclature of the eight classes adopted here follows Eschmeyer's Catalog of Fishes). 'Reptiles' are treated here as comprising five lineages: Squamata, Testudines, Crocodylia, Rhynchocephalia, and Aves (modern dinosaurs). Accordingly, Craniata includes 15 lineages, which we treat as canonical in this study.
Of these 15 lineages, 11 occur in Brazil. Cladistii is restricted to African rivers; Coelacanthi is found only in parts of the Indian Ocean near Africa and SE Asia; Rhynchocephalia is limited to islands in northern New Zealand; and Petromyzonti occurs only in temperate regions of both hemispheres — being the only one among these four to also occur in the New World. Within this context, South America holds the highest global concentrations of birds, mammals, and amphibians, as shown in the maps below. Among New World, the highest diversity in clades are in Argentina by records simultaneous of Dipneusti and Petromizontida.
34.1 MYXINI ‣ (6/)91 spp. worldwide in a single family - Myxinidae - and order (CTFB/Eschmeyer's CF/20260217). (3/)5 spp. occur in Brazil (Myxini, Nov 16, 2024), two endemic (Intreasures/20260217). Genera no recorded in Brazil includes Rubicundus (4, in the isolated collection: Tasman Sea, Galapagos/Ecuador, Taiwan, and off SE USA, Fernholm & Quattrini, Copeia, 2008), Notomyxine (1, Argentina, Chile and Uruguay, Lorenz et al., Biology, 2014), and Neomyxine (2, coasts of New Zealand, Wikipedia).
34.2 PETROMYZONTI ‣ (11/)49 spp. in three families a single order (unknown in Brazil), Petromyzontiformes (Eschmeyer's CF/20260217). For data from all species described until 2011, see Renaud (FAO 2011). Petromyzonti have the highest number of chromosomes (164–174) among vertebrates (Wikipedia).
GEOTRIDAE ‣ (1/)2 spp. (Wikipedia), both anadromous, Geotria australis J. E. Gray, 1851 and G. macrostoma Burmeister, 1868, from cold waters of E, C & S Argentina (including Malvinas), C & S Chile, S Uruguay, SW & SE Australia and all New Zealand (MAP). In South America, Geotria occurs in the central-southern regions of Chile and Argentina, with records in the lower Paraná River, near Buenos Aires, and along the coast of Uruguay, less than 350 km from the Brazilian coast (MAP).
MORDACIDAE ‣ (1/)3 spp. (Wikipedia), Mordacia mordax J. Richardson, 1846 (anadromous, SE Australia), M. lapicida J. E. Gray, 1851 (anadromous, C Chile), and M. praecox Potter, 1968 (resident and non-feeding, SE Australia), from C Chile and SE Australia (MAP).
PETROMYZONTIDAE ‣ (9/)44 spp. (Fish Base), exclusively from northern Hemisphere, in Caspiomyzon (3, in Greece, Volga/Russia, Ural/Russia-Kazakhstan, Kura/Azerbaijan and Sefid/Iran systems), Entosphenus (6, Lake Cowichan and Mesachie Lake/Canada and Klamath/USA systems for five smaller-range species, E. tridentatus J. Richardson, 1836 distributed along the northern Pacific of Baja California to Hokkaido in Japan), Eudontomyzon (5, 4 in SE Europe, 1 in NE Asia, in Yalu/China-North Korea system), Ichthyomyzon (6, Hudson Bay to the Gulf of Mexico in USA), Lampetra (10, E North America and Europe, with a notable three species endemic to Portugal), Lethenteron (7, North America, Asia and Europe), Occidentis (4, Alaska to California in Pacific coast), Petromyzon (1, skirting the North Atlantic from Florida to NW Africa), and Tetrapleurodon (2, Pacific drainages of Celio, Jacona, Duero, Zamora and Lerma rivers, and Lake Chapala in Jalisco and Michoacán states, Mexico).
34.3 ELASMOBRANCHII ‣ (65:212/)1,256 spp. worldwide in 13 orders (Eschmeyer's CF/20260217). (37:83/)181 spp. occur in Brazil (CTFB/Chondricthyes) in 10 orders (Hexanchiformes, Orectolobiformes, Lamniformes, Carcharhiniformes, Squaliformes, Pristiophoriformes, Squatiniformes, Torpediniformes, Rajiformes, and Myliobatiformes — excludes Chimaeriformes, treated here as Holocephali), being (4/)23 freshwater, 157 marine, and one amphidromous. 27 species are endemic to Brazil (Intreasures/marine, Intreasures/freshwater — 13 marine and 14 freshwater in Potamotrygonidae).
The orders in Eschmeyer's CF/20260217 and CTFB are consistent, except for Rhinopristiformes in former reference, which CTFB recognizes as Pristiformes, with the CTFB version placing the family Rhinobatidae in Rajiformes (and not in Rhinopristiformes/Pristiformes).
Two orders of Elasmobranchii do not occur in Brazil: Heterodontiformes (1:1/8, Indian and Pacific Ocean, 3 of these on the Pacific coast of the New World: Heterodontus franciscii Girard, 1855 in California, Baja California and possibly Ecuador and Peru, H. mexicanus from Mexico to Peru, and H. quoyi Fréminville, 1840 from Peru and Galápagos), and Pristiophoriformes (1:2/10, W Indian Ocean from Arabia to South Africa, east up to Mauritius, around Australia, Japan to Taiwan, Philippines, and off coast of Bahamas to Cuba and Puerto Rico in Atlantic Ocean). 162 marine species in Mexico (FishBase/Mexico).
Freshwater Elasmobranchii belongs two families (Lucifora et al., CBM, 2015): Potamotrygonidae in South America (Amazon, Río de la Plata, Orinoco, Magdalena, Maracaibo, Parnaíba, and the Guianas Basins, 6/40 in freshwater, 29 in Brazil, 14 endemic) and Dasyatidae in Africa (Congo, Niger, Sanaga, and Cross) and SE Asia (Mekong, Irrawaddy, Maekhlong, Chao Phraya, and Bornean, Sumatran and peninsular Malaysian rivers). Potamotrygonidae includes a single marine species, Styracura schmardae (Werner, 1904), widely and collected in Brazil.
34.4 HOLOCEPHALI ‣ (3:6/)60 spp. in a single order worldwide (Eschmeyer's CF/20260217), (3:4/)6 in Brazil in all three families of this class (CTFB/Chondrichthyes), one endemic to country (Intreasures). Two genera do not occur in Brazil: Chimaera and Neoharriotta (3, Díez & Mugerza, Journal of Ichthyology, 2017).
34.5 ACTINOPTERI ‣ (538:5,094/)36,042 spp. worldwide in 53 orders worldwide (Eschmeyer's CF/20260217 Actinopteri minus data from remaining 7 fish taxa) and (222/1,352)5,030 spp. native in Brazil by CTFB/Actinopterigii.
Six species and two genera native to Brazil are not recorded from from the broader CTFB search; these have been added here to the national total. Accordingly, we adopt a final count of (221:1,300/)4,674 native Actinopteri species in Brazil. These are: Eustomias lucenae (Stomiidae, SEE), Pseudocyttus maculatus (Oreosomatidae, new genus for Brazil, SEE), Petrotyx sanguineus (Bythitidae, new genus for Brazil, SEE), Chaunax pictus (Chaunacidae, SEE), Epigonus macrops (Epigonidae, SEE), and Pontinus rathbuni (Scorpaenidae, SEE).
FRESHWATERS
Ostariophysi is the second largest superorder of fishes, formed almost exclusively by freshwater species, with only about 120 marine species, along 102 families, 1372 genera, and 11,883 species, thus containing approximately 30% of the known fish species in the world and almost 70% of the freshwater species, in 5 orders: Gonorhynchiformes, Cypriniformes, Siluriformes, Gymnotiformes, and Characiformes. The size range among Ostariophysi is remarkably diverse, ranging from tiny species such as Paedocypris progenetica (Kottelat, Britz, Tan & Witte, 2006), with an adult length of 7.9–10.3 mm, , to very large species such as Brachyplatystoma filamentosum (Valenciennes, 1840), with an adult length reaching 3.6 m (Oliveira, Taxonomy, 2025).
Fish biodiversity and conservation in South America (R. Reis et al., Journal of Fish Biology, 2016) makes a significant contribution to the understanding of fish diversity, particularly Actinopteri, in both freshwater and marine environments of South America. Neotropical freshwater fish (NFF) fauna includes at least 6,345 valid species distributed throughout the rivers, lakes and wetlands of tropical South America, America Central south of the Trans-Mexican Volcanic Belt, and the Greater Antilles. This diversity represents about one-sixth (18%) of all c. 36,000 valid fish species globally, or about one in 11 (c. 9%) of all the known vertebrate species who live on the Earth today (Albert, JS et al, Scientific Data, 2025).
In terms of freshwater fish species, considering that the overwhelming majority belong to Actinopteri, Brazil leads globally with around 3,742 spp. (Fish Base/20260223), ahead of China in second place (1,616) and well ahead of other notable countries such as Mexico (582, Fish Base), Australia (360), and Indonesia (1,250). All freshwater fishes from Brazil are Actinopteri except 29 Elasmobranchii in Potamotrygonidae, and Lepidosiren paradoxa Fitz, 1836 (Dipneusti).
Of the 74 families of Actinopteri with freshwater species in South America, 66 are found in Brazil. The remaining 8 include: four families mostly restricted to Argentina and Chile (some also occurring in Oceania), one Andean family, two widely distributed families, and two primarily tropical families. These are:
Cyprinodontidae (9-10/103, Cyprinodon up to NE Colombia, Venezuela, possibly in Guyana; Orestias (45) in 2,800–4,600 m in the Andes of C & S Peru, W Bolivia, and NE Chile; Pseudorestias (1) in N Chile; Yssolebias in Rio Madalena in Colombia),
Gobiesocidae (53/189, seven freshwaters in Gobiesox from North America to Venezuela and Peru; Gobiesox includes saltwaters in Brazil),
Astroblepidae (1/82, Panama to Bolivia, mainly in Andean region, all freshwater),
Anguillidae (1/15, Anguilla rostrata Lesueur, 1817, from Greenland up to Colombia and Venezuela, MAP, PDF),
Nematogenyidae (1/1, C Chile),
Diplomystidae (2/7, W Argentina and E Chile),
Percichthyidae (7/23, Australia, Argentina and Chile), and
Galaxiidae (7/66, mainly Australia, New Zealand and Tasmania, (3/)7 spp. in Argentina and Chile, and one in South Africa).
In these 8 families, (14/)154 spp. occur in South America (4/5 in Austroblepus or Orestias), being eight only in Argentina and Chile, three only in Colombia and Venezuela, and three wider (Astroblepus, Orestias, Gobiesox).
The highest altitudinal record (1,944 m) for a freshwater fish in Brazil is for Psalidodon cf. scabripinnis (Jenyns, 1842), a Characidae collected in streams of the Serra da Mantiqueira that drain into the Paraíba do Sul River basin. Herzensteinia microcephalus (Herzenstein, 1891), a Cyprinidae, was recorded on the northern slope of Mount Tanggula on the Qinghai-Tibet Plateau in the Himalayas at 5,350 m asl, being the highest-altitude fish occurrence known (Soares Gudes, G.H. et al, Zoologia, 2025).
MARINE
When it comes to marine fish, mainly Actinopteri, Brazil’s diversity is paradoxically underrecognized compared to many other countries, ranking only 21st worldwide (1,269, Fish Base/20260223) and ranking below Australia (4,677), Japan (3,869), Indonesia (3,646), Philippines (3,123), Taiwan (2,756), Papua New Guinea (2,601), New Caledonia (2,345), Mexico (2,132, FishBase/Mexico), USA (2,111), South Africa (1,948), China (1,808), Vietnam (1,742), India (1,679), Palau (1,530), Mozambique (1,450), Thailand (1,407), Malaysia (1,366), Fiji (1,265) and Panama (1,262), and ahead Colombia (1,229) and Micronesia (1,227).
For large marine fishes of Brazil (mainly Actinopteri), see Grandes Peixes Oceânicos da Costa Brasileira (Alfredo Carvalho Filho's Lab, BOOK, 2020, 107 spp.). 225 marine spp. occur in Saint Peter and Saint Paul's Archipelago off Brazil (Carvalho-Filho et al., Journal of Fish Biology, 2020). 36 deep-sea fishes occur in northern coast of Brazil (Klautau et al., Neotropical Ichthyology, 2020). 273 spp occur in Vitória-Trindade Seamount Chain (Pinheiro et al., Plos One, 2015). 250 spp. occur in Fernando de Noronha Island (Pimentel et al., Neotropical Ichthyology, 2020). For 111 endemic reef-fishes in SW Atlantic, see Pinheiro et al. (Diversity and Distribution, 2018).
ENDEMIC
Brazilian Actinopteri includes 2,488 endemic species (Intreasures/20260227), being 2,351 freshwater, 107 in shallow salt sea, and 30 in deep sea below 200m.
For endemic freshwater species, Brazil has 2,351 spp. (Intreasures/20260227, excluding one Lepidosirenidae abd 29 Potamotrygonidae), more than the sum of the joint endemic species of China and the USA, the following in the list, and about 7✕ those of Mexico. 492 of these are Loricariidae, 435 Characidae, 296 Rivulidae and 138 Cichlidae. In addition, there are 123 genera (Intreasures/20260227) endemic (in 26 families, mainly Loricariidae), with notable emphasis on Hypsolebias (52, Rivulidae), Pareiorhaphis (28, Loricariidae), Parotocinclus (27, Loricariidae), Cynolebias (22, Rivulidae), Neoplecostomus (19, Loricariidae), and Aspidoras (18, Callichthyidae). 34 of these genera are Loricariidae and 28 are Characidae.
Mexico has 42 endemic genera of Actinopteri in 17 families, 16 of which are Goodeidae, 7 in Leuciscidae, and 2 Cyprinidae. Of the 316 spp. of freshwater Actinopteri endemic to Mexico, 46 are Goodeidae, 27 Cyprinodontidae and 70 are Poeciliidae.
Among New World countries, only USA, Chile, and Brazil host endemic families of freshwater fishes, all within Actinopteri. In Brazil, Tarumaniidae (1/1, Tarumania walkerae Pinna, Zuanon, Py-Daniel & Petry, 2017) and Spintherobolidae (2/5) are endemic family. In Chile, Perciliidae (1/2) and Nematogenyidae (1/1) are endemic, while in the USA, the endemic families include Amblyopsidae (6/9), Aphredoderidae (1/1), and Elassomatidae (1/7). Lacantuniidae was once considered endemic to Mexico but has also been recorded in Guatemala (Quintana et al., CheckList, 2019).
All families with endemic genera of freshwater fish in any country in South America are Actinopteri and have them in Brazil, except: Chenuchidae (only in Peru), Lebiasinidae (only in Guyana), Galaxiidae, Nematogeniidae and Perciliidae (these only in Chile).
For endemic marine Actinopteri, Brazil have only 104 in swallow water and 30 in deep sea. Brazil has the 10th position worldwide in overall endemic marine fishes, with Australia (933), Indonesia (241) and Japan (240) leading in this regard, and with USA (220) and Mexico (162) also in front of us (Intreasures, November 16, 2024). By marine endemic genera in Actinopteri, Brazil had only four of them (Storrsia, Akko, Asarcenchelys and Leucogrammolycus) in 4 families. USA has five genera in five families, and Mexico has 11 genera in nine families. Asarcenchelys (Ophichtidae) occurs from Pará to Bahia state coasts (A. Carvalho-Filho et al. Marine Biodiversity Records, 2011).
INVASIVE IN BRAZIL
A recent study detected 352 spp. of non-native fish from some region of Brazil, being 255 translocated and 97 exotics (Silva Rocha, Biological Invasions, 2023).
WORLD RECORDS
A maximum depth record for Actinopteri has been set at 8336m deep in the Izu-Ogasawara Trench (NW Pacific Ocean) represented by video imagery of a snailfish probably Pseupodoliapris belyaevi or a new, endemic species since no physical specimen was captured (Jamieson, AJ et al, Deep Sea Research Part I Oceanographic Research Papers, 2023). Two of three smallest fishes (possibly the smallest worldwide) are Brazilian Leptophilypnion (Eleotridae, fishes only 9mm lenght, by Wikipedia), genus with only two species, endemic to the states of Amazonas and Pará each, but the genus is not recognized as endemic to Brazil in Intresures.
TAXONOMY and ORDERS
Based on FishBase/Search, Eschmeyer's CF/Table/20260227, and CTFB/Actinopteri, there are 12 orders of Actinopteri not recorded in Brazil. Gymnotiformes, Symbranchiformes and Osteoglossiformes do not occur in North America.
ACTINOPTERI PHYLOGENY, WITH 3 BASAL ORDERS NOT RECORDED IN BRAZIL
ACIPENSERIFORMES
Two primitive families. For a excellent sourve among this order, see Pond Life.
Polyodontidae ‣ two species, Polyodon spathula Walbaum, 1792 from the Mississipi Basin and adjacent flows, USA (MAP) and Psephurus gladius Martens, 1862, only Yang-Tse river in China, extinct (MAP).
Acipenseridae ‣ (4/)25 spp. in Acipenser (Aleutian islands to NW Mexico, inland in some stremas in NW USA and SW Canada-2, Quebec to Lousiana and several strems inland E USA and E Canada-2, Mississipi Basin, Canadian rivers, Greater Lakes-1, Hainan to Sakhalin, inland in Yang Tze-River and , Amur river in NE China and SE Russia and Japan-4, Iceland, Kola peninsula to Marocco, northern Mediterranean from Algeria to Turkyie, Black Sea and Caspian Lake shores, inland in Pó, Danube, Dniepre, Don, Volga, Ural, Kura, Ghezel Ozan in N Iran, Dvina, Ob and Yenissei rivers-7, and Artic and Asian Russia into Kazhakhstan and Baikal Lake-1), Huso (2, one in Black, Azov, Caspian and Adriatic sea basins from N Italy to European Russia and N Iran/Sturgeon Web, and one in the Amur Basin BE China, SE Russia, North Korea, Sakhalin and Hokkaido island in Japan/Sturgeon Web), Pseudoscaphyrhynchus (3, endemic to the extinct Aral Lake, currently in Turkmenistan, Uzbekistan, Tajikistan and perhaps Afghanistan/Wikipedia) and Scaphyrhynchus (3, S. albus S. A. Forbes & R. E. Richardson, 1905 and S. platorynchus Rafinesque, 1820 from Montana to Louisina along Mississipi river and smal tributaries, and S. suttkusi J. D. Williams & Clemmer, 1991 in Alabama river in Alabama state).
LEPISOSTEIFORMES
A single living family, 7 spp. in two genera from North America, America Central and Caribbean. Brazilian fossils in PaleoZoo/Leptososteiformes.
Atractosteus (3) ‣ A. spatula Lacépède, 1803 (S USA), A. tristoechus Bloch & J. G. Schneider, 1801 (W Cuba and the Isla de la Juventud) and A. tropicus T. N. Gill, 1863 (S Mexico to Costa Rica).
Lepisosteus (4) ‣ L. oculatus Winchell, 1864 (Ontario to Texas in eastern half of USA), L. osseus L., 1758 (SE Canada to New Mexico and E Mexico), L. platostomus Rafinesque, 1820 (Montana to the west and the Ohio River to the east, southwards to the Gulf Coast) and L. platyrhincus DeKay, 1842 (Georgia and Florida).
AMIIFORMES
Composed only by Amiidae and Amia calva L., 1776, from the E USA (from Minnesota to Texas and Florida) and extreme SE Canada. Brazilian fossils in PaleoZoo/Amiiformes.
LEPIDOGALAXIIFORMES
Only one sp., Lepidogalaxias salamandroides Mees, 1961, endemic to extreme point of W Australia, in a very small range.
ANABANTHIFORMES
8 families (26/)283 spp., from Africa and tropical Asia from India to Philippines (Eschmeyer's CF/20260223).
SALMONIFORMES
(15/)278 spp. worldwide, composed by the family Salmonidae (11/264, Balcans to Korea and Canada to Mexico, only Oncorhynchus in latter), Esocidae (3/11, Canada. USA, Asia to the Amur and Europe) and Umbridae (1/3, Canada. USA, Europe and Asia).
OSMERIFORMES
4 families and (17/)42 spp., three from E Russia to New Zealand, and Osmeridae in New World, from North Atlantic and North Pacific Oceans, as well as rivers, streams and lakes in Europe, North America and NE Asia. Excludes Argentiniformes.
PERCOPSIFORMES
Three families and (8/)16 spp., in Percopsidae (1/2), Amblyopsidae (6/9, USA) and Aphedoderidae (1/4, exclusively from the USA)., colletively from Alaska to Quebec and southward to Missouri and Kentucky, E USA, primarily lowlands of Atlantic drainage from Long Island southward, Gulf of Mexico slope, Mississippi Valley, and part of Great Lakes drainage.
HIODONTIFORMES
Only Hiodontidae and (1/)2 spp. from C & E USA and SE Canada (primarily Mackenzie, Saskatchewan, Mississippi, and St. Lawrence river systems).
CYPRINIFORMES
(321/)3,268 spp. in six families, only two in New World: Catastomidae (15/85 spp., almost endemic to North America except species in Russia and China one each) and Cyprinidae (162/1780), from Old World and (53/)286 spp. New World, 52 of them endemic, from Canada to south Mexico, and Phoxinus also in Eurasia. In USA, diversities includes 119 in Mississippi Basin, 80 in Cuberland and Tennesseee Basins, 47 in Mobile Basin, 31 in Hudson Bay Basins, 16 in Colorado Basin, 43 in Rio Grande Basin; in Mexico, diversities includes Panuco (11), Balsas (2), Papaloapan (1, Atlantic southermost record, Veracruz) and Atoyac (1, Pacific southermost record, Oaxaca), both with Hybopsis species (Winfield & Nelson, BOOK, 1991). (17/)77 spp. in Mexico (FishBase), 50 endemic (and 7 endemic genera, Fish Base).
GONORYNCHIFORMES
(4:7/)41 spp., in Gonorrhynchidae (1/5, only G. gonorhynchus L., 1766, cited for the New World, in Chile, rare in southern Atlantic, e.g., Santa Helena), Phractolaemidae (1/1, Niger Delta and Malebopool and Zaire systems), Kneriidae (4/33, Africa) and Chanidae (1/1, Chanos chanos Forsskål, 1775, tropical and subtropical Indian and Pacific, rare in eastern Pacific from southern California and Mexico to Peru).
GALAXIFORMES
Single-family order with (7/)66 spp., mainly in Australia, New Zealand and Tasmania, but three genera occur in diverse areas in southern Hemisphere, i.e. Aplochiton (3, exclusive to Argentina and Chile), Brachygalaxias (2, endemic to Chile), and Galaxias (46, 42 in Oceania, 3 in South America and 1 in South Africa).
■ endemic families in New World: Polyodontidae (1/1, USA), Amblyopsidae (6/9, USA), Aphredoderidae (1/1, USA), Elassomatidae (1/7, USA), Nematogenyidae (1/1, Chile), Perciliidae (1/2, Chile), Spintherobolidae (2/5, Brazil) and Tarumaniidae (1/1, Brazil).
34.6 CLADISTII ‣ 14 spp. in two genera (Erpetoichthys and Polypterus, Eschmeyer's CF/20260217) within a single family (Polypteridae) in order Polypteriformes, in freshwater bodies from N Senegal to SW Tanzania, north along Nile river up to Egypt, highly centered in Congo River system (Moritz & Britz, Ichthyological Exploration of Freshwaters, 2019).
34.7 DIPNEUSTI ‣ (3/)6 spp. along three families in a single order Ceratodontiformes (Eschmeyer's CF/20260217): Neoceratodontidae (1/1, Neoceratodon forsteri Krefft, 1870, endemic to coastal basins in Queensland, Australia), Protopteridae (1/4 in Protopterus, tropical Africa) and Lepidosirenidae (1/1, Lepidosiren paradoxa Fitz, 1836, from Brazil and several adjacent countries except Peru and Uruguay).
Lepidosiren genome (about 91 Gb, roughly 30 times the human genome) is the largest animal genome sequenced so far and more than twice the size of the Australian (Neoceratodus forsteri) and African lungfishes owing to enlarged intergenic regions and introns with high repeat content (Schartl, M. et al, Nature, 2024).
34.8 COELACANTHI ‣ only two species in a single genus and family: Latimeria chalumnae Smith, 1939, from Kenya, Tanzania, Mozambique, Madagascar, Comoros Archipelago up to South Africa; and L. menadoensis Pouyaud et al., 1999, from north of Sulawesi to N New Guinea island in Indonesia (Kadashuman, Scientific Reports, 2020).
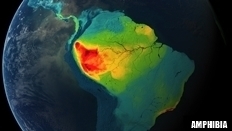
WORLD HOTSPOT OF AMPHIBIAN DIVERSITY (BM)
34.9 AMPHIBIA ‣ (79:597/)9,007 spp. worldwide in three orders (Advanced Checklist of all species and genera/20260217), being (9:70/)837 in Caudata, (60:496/)7,938 in Anura and (10:32/)232 in Gymnophiona.
Brazil has (27:121/)1,253 spp. in all three orders, being (23:108/)1,208 in Anura, (4:13/)40 in Gymnophiona (36 non-Caecilia) and (1:1/)5 in Caudata (AW/Brazil, 20260217).
Colombia has 42 spp. of Gymnophiona (16 non-Caecilia), 28 of Caudata and 795 of Anura (AW/Colombia). Worldwide, Colombia leads in Gymnophiona (42), Brazil in Anura (1,208), and USA in Caudata (232). Mexico includes 265 Anura, 154 Caudata and 3 Gymnophiona. For list updates see UPDATES 2025. For some curiosities of Amphibia, see Amphibia Facts.
NEW WORLD CONTEXT
In New World occur 43 families, being 30 Anura, 8 Caudata and 5 Gymnophiona. All New World families occur in South America except the Anuran Ascaphidae (1/2, Canada to USA), Rhinophrynidae (1/1, Texas to Costa Rica), and Scaphiopodidae (2/7, Canada and USA south to S Mexico), CaudataN Amphiumidae (3, endemic to USA), Rhyacotritonidae (4, endemic to USA), Ambystomatidae (30, USA up to Canada and Mexico), Sirenidae (5, USA up to Canada and Mexico), Salamandridae (132), Cryptobranchidae (4) and Proteidae (9), the last three also in Old World.
AMPHIBIAN FAMILIES IN SOUTH AMERICA NOR RECORDED IN BRAZIL
Five South American amphibian families do not occur in Brazil: Telmatobiidae (Anura, 1/63), with Telmatobius from Ecuador to Argentina and Chile; Batrachylidae (Anura, 4/12), restricted to Chile and Argentina; Calyptocephalellidae (Anura, 2/15), endemic to central Chile; Rhinodermatidae (Anura, 2/3), from Argentina and Chile; and Dermophidae (Gymnophiona), the only family of the order to occur in Mexico, with 4 genera, two in Africa, Gymnops (2) in America Central, and Dermophis (7) from America Central, centered in Costa Rica, but two extend to S Mexico, and two to NW Colombia.
ENDEMIC TAXA
In endemic species, Brazil has 865 spp. (Intreasures/20260217). In the Mexico ✕ Brazil duel among endemic species we have Caudata with 134 ✕ 4, Gymnophiona with 1 ✕ 26 and Anura with 172 ✕ 835, respectively. 195 of the 245 USA endemic amphibians are Caudata. Mexico, Peru, Colombia and Brazil are among the few countries in the World with endemic species in the three orders of Amphibian.
In endemic genera, in Brazil there are 34 genera: 3 in Gymnophiona (in Siphonopidae and Typhlonectidae) and 31 in Anura (in 13 families: Brachycephalidae, Bufonidae, Cyclorhamphidae, Craugastoridae, Eleutherodactylidae, Hemiphractidae, Hylidae, Hylodidae, Leptodactylidae, Microhylidae, Neblinaphrynidae, Odontophrynidae and Strabomantidae). Emphasis on the genera Brachycephalus (42), Bokermannohyla (32) and Cycloramphus (28). Mexico there are nine endemic genera (3 in Hylidae and 6 in Plethodontidae), with the notable presence of Thorius (29). All eight genera of Amphibia endemic to the USA are from Caudata. Colombia has 5 endemic genera in Strobomantidae (2), Dendrobatidae (1), Centrolenidae (1) and Siphonopidae (1). Dryadobates (Aromobatidae), endemic to E Brazil, was described in 2025 (ASW).
ANURA
Brazilian diversity by family (Jan 24, 2026): Allophrynidae (1/3), Alsodidae (1/1), Brachycephalidae (2/82), Caligophrynidae (1/1), Ceuthomantidae (1/2), Craugastoridae (9/67), Eleutherodactylidae (2/13), Neblinaphrynidae (1/2), Bufonidae (9/96), Centrolenidae (4/17), Ceratophryidae (2/6), Cycloramphidae (2/37), Aromobatidae (3/47), Dendrobatidae (5/26), Hemiphractidae (4/21), Hylidae (20/390), Hylodidae (4/48), Leptodactylidae (13/194), Microhylidae (12/56), Odontophrynidae (3/52), Phyllomedusidae (7/42), Pipidae (1/5) and Ranidae (1/1), totaling (23:108/)1,207 spp.
Colombian diversity by family (Jan 24, 2026): Brachycephaloidea undeterminate family (1/1), Craugastoridae (8/267, 222 in Pristimantis), Eleutherodactylidae (3/8), Bufonidae (7/78), Centrolenidae (10/80), Ceratophryidae (1/2), Aromobatidae (2/17), Dendrobatidae (13/99), Hemiphractidae (4/28), Hylidae (13/132), Leptodactylidae (9/40), Microhylidae (6/20), Phyllomedusidae (5/17), Pipidae (1/5) and Ranidae (1/1). Brachycephaloidea undetermined family (1/1) includes a single species, Atopophrynus syntomopus Lynch and Ruiz-Carranza [fr], 1982, known only from Antioquia departament, with uncertain familial affiliation and potentially representing an endemic family of the country. Nine families occur in Brazil but not in Colombia: Allophrynidae, Alsodidae, Brachycephalidae, Caligophrynidae, Ceuthomantidae, Neblinaphrynidae, Cycloramphidae, Hylodidae, and Odontophrynidae. In only five families does Colombia exceed Brazil in the number of genera and/or species: Craugastoridae (8/267 ✕ 9/67), Eleutherodactylidae (3/8 ✕ 2/13), Centrolenidae (10/80 ✕ 4/17), Dendrobatidae (13/99 ✕ 5/26), and Hemiphractidae (4/28 ✕ 4/21).
Unbrazilian genera in South America belongs Alsodidae, Aromobatidae, Batrachylidae, Bufonidae, Calyptocephallelidae, Centrolenidae, Ceratophryidae, Craugastoridae, Dendrobatidae, Eleutherodactylidae, Hemiphractidae, Hylidae, Rhynodermatidae, Strabomantidae and Telmatobiidae. Batrachylidae, Calyptocephallelidae, Rhynodermatidae and Telmatobiidae do not occur in Brazil. In Craugastoridae, Bufonidae, Hylidae, Eleutherodactylidae and Hemiphractidae Brazil has the largest diversity of genera and species.
The large family Eleutherodactylidae has 4 genera (one of them endemic to Brazil). Eleutherodactylus has 204 spp., only one in South America, E. johnstomei Barbour, 1914. Large genera few represented in Brazil includes Hyloxalus (63, Dendrobatidae, only two in Brazil) and Pristimantis (569, only 35 in Brazil). 1/3 of all Colombian Anura are Pristimantis.
Only two amphibians in the world are venomous, both Hylidae endemic to E Brazil: Nyctimantis brunoi Miranda-Ribeiro, 1920, and Corythomantis greeningi Boulenger, 1896 (NHM). Brachycephalus dacnis Toledo, Botelho, Carrasco-Medina, Gray, Ernetti, Gama, Lyra, Blackburn, Nunes, and Muscat, 2024 from coastal forests from NE São Paulo and SW Rio de Janeiro states in SE Brazil is among the smallest known vertebrate (Toledo et al., PeerJ, 2024). Sperandei, V.F. et al. (Biota Neotropica, 2024) discuss Anura in caves in Brazil, citing 54 species that have already been collected in caves. Oreobates antrum Vaz-Silva, Maciel, Andrade and Amaro, 2018 has only been found inside caves, in the states of Goiás and Tocantins. However, there are no known records of troglobic Anura worldwide.
CAUDATA
All Caudata from southern Mexican Plateau are Bolitoglossines. Mexican diversity of Caudata (the second worldwide, SEE) is concentrated in Plethodontidae (15/141, 6 genera endemic), and the only exceptions are Ambystomidae (1/11), a single Sirenidae (Siren nettingi Goin, 1942) and a single Salamandrideae (Notophthalmus meridionalis Cope, 1880).
42 spp. of Caudata occur in South America, in Oedipina (4 in region, 3 in Colombia - none endemic - and adjacent countries Ecuador and Panama, and one endemic to Ecuador) and Bolitoglossa (38), with one from Panama to Colombia, 4 endemic to Venezuela, 3 from Venezuela to Colombia, 16 endemic to Colombia, 3 from Colombia to Ecuador, one from Ecuador to Peru, 4 endemic to Peru, 4 endemic to Brazil, and two widely species, B. medemi Brame and Wake, 1972 from Panama to Ecuador, and B. aff altamazonica Cusi, Gagliardi-Urrutia, Brcko, Wake and von May, 2020, from Venezuela to Bolivia and N Brazil.

NEOTROPICAL GENERA OF BOLITOGLOSSINES BY REGION
Data in Jaramillo et al. (Molecular Phylogenetics and Evolution, 2020) suggests the existence of 36 potential new species of Bolitoglossa in the Amazon. If confirmed, the current diversity of the genus will increase in Colombia (25 to 26, excluding B. altamazonica Cope, 1874), Ecuador (8 to 17, excluding B. altamazonica), Peru (6 to 28) and Brazil (5 to 8, 7 endemic and one shared with Peru, excluding B. altamazonica).
GYMNOPHIONA
In Gymnophiona, all New World genera occur in Brazil except Dermophis (7, Mexico to Colombia), Amazops (1, endemic to Ecuador), Gymnopis (2, Mexico to Panama) and Epicrionopis (7, Venezuela to Peru, possibly in Brazil). Brazil has the highest species diversity among all New World genera of Gymnophiona except Caecilia (1 in Costa Rica, 5 in Panama, 4 in Venezuela, 27 in Colombia—18 endemic, 18 in Ecuador, 7 in Peru, 1 in Bolivia, 1 in Guyana, 3 in Suriname, 2 in French Guiana, and 3 in Brazil), Oscaecilia (3 in Colombia, 2 in Panama, and 1 in Guyana, Brazil, Peru, and Ecuador), Dermophis (unknown in Brazil), Gymnophis (unknown in Brazil), Amazops (unknown in Brazil), Epicrionops (unknown in Brazil), and Typhlonectes (Colombia has one more species).
Siphonopidae is paraphyletic with respect to Dermophiidae due to the placement of Parvicaecilia (Microcaecilia nicefori Barbour, 1924, endemic to Colombia), by Acosta-Galvis et al. (ZooKeys, 2019).
Hopkins & Brodie (Herpetological Monographies, 2015) lists 144 spp. in 28 families of Amphibia in saline habitats, some collected in Brazil, including the single record of this feature at Gymnophiona, the Brazilin endemic Atretochoana eiselti Taylor, 1968. A. eiselti is also the largest lungless tetrapod worldwide (Hoogmoed et al., Bol. Mus. Para. Emílio Goeldi, 2011).
■ endemic families in New World: Amphiumidae (Caudata, 1/3, USA), Calyptocephalellidae (Anura, 2/5, Chile), Cyclorhamphidae (Anura, 3/37, Brazil), Neblinaphrynidae (Anura, 2/2, Brazil), Rhyacotritonidae (Caudata, 1/4, USA).
34.10 RHYNCHOCEPHALIA ‣ only extant species, Sphenodon punctatus Gray, 1842 (Wikipedia), restricted of small islands aroung North Island in New Zealand. This lineage is the highest degree of national endemism in a country among the Craniata and one of the largest in Metazoa.
34.11 SQUAMATA ‣ (70:1,179/)12,324 spp. worldwide (Repfocus/Lizards | Repfocus/Amphisbaenia | Repfocus/Snakes/20260217, 'lizards' highly paraphyletic) in nine high lineages. Brazil includes 778 spp. (SAU | SER | AMP) in 165 genera (SAU | SER | AMP) at 25 families (SAU | SER). At this date, Brazil has the third largest diversity of Squamata worldwide, after Australia (1,020) and Mexico (897), and ahead Indonesia (764). Brazil has the fifth largest diversity in endemic Squamata (418, Intreasures), after Australia (1,011), Mexico (578), India (478), Madagascar (429), and ahead Indonesia (375). In families, Brazil and Mexico has 26, Colombia 24, USA only 11. Among endemic genera in New World, Brazil has 40 (in seven families, including 13 in snakes, all Colubridae), Mexico 18 (in six families, 14 in snakes, in 3 families), Colombia only one (in Gymnophthalmidae) and USA has 8, all in snakes (Intreasures).
38 families of Squamata occur in New World, 25 in Mexico, 13 do not occur in Brazil: Rhineuridae (1/1, Florida and Georgia, USA), Cadeidae (1/1, W Cuba), Leiocephalidae (1/24, Jamaica, Cuba, Bahamas, Hispaniola, Puerto Rico), Dibamidae (23, 1 C in Mexico, 22 in SE Asia), Eublepharidae (6/48, S USA to Panama, Old World), Xanthusiidae (3/38, SW USA to Panama), Bipedidae (1/3, NW & C Mexico), Helodermatidae (1/5, W USA to S Mexico), Xenosauridae (1/14, E Mexico to Honduras), Phrynosomatidae (9/178, W to E USA to Costa Rica), Crotaphytidae (2/12, W & C USA to N Mexico), Corytophanidae (3/11, C Mexico to N Ecuador and Venezuela) and Loxocemidae (1/1, Pacific coast from C Mexico to NW Costa Rica). All endemic families in New World belong Amphisbaenia clade: USA, Mexico and Cuba one endemic each. In all Brazilian families, the own Brazil has the first diversity of genera in New World (shared or isolated) except Anguidae, Iguanidae, Liolaemidae and some families in Serpentes.
Mexico harbors an extraordinary diversity of Squamata. The basal lineage Dibamia is represented by a basal, relictual, and highly disjunct member in northwestern Mexico. In addition, Xantusiidae, Helodermatidae, Anniellidae, and Crotaphytidae are families whose geographic centers lie in Mexico. Bipedidae is endemic to the country. Phyllodactylidae, Scincidae, Anguidae, and Iguanidae exhibit explosive radiations of diversity within Mexico. In contrast, Mexico lacks native representatives of Gekkonidae and Alopoglossidae; the diversity of Amphisbaenia and Gymnophthalmidae is extremely low, and there are five families of Iguania that are exclusive to South America.
For all 67 spp. of marine Squamata (sea snakes and marine iguane, all unknown in Brazil), see Bertolero et al. (EOLSS, 2009 | Books Google).
DIBAMIA
A single family.
DIBAMIDAE ‣ 29 spp. in two disjunct genera (Repfocus/Dibamidae), Anelytropsis (1, Mexico) and Dibamus (26, Vietnan, Cambodia, Malaysia, Indonesia and Philippines up to NW New Guinea), almost legless except male of some species, with small flap-like hind limbs.
GEKKOTA
Families not recorded in New World are Diplodactylidae (24/201, Australia, New Zealand and New Caledonia), Pygopodidae (7/48, Australia and New Guinea) and Carphodactylidae (7/34, Australia). 17 genera in New World, 11 in Brazil (the largest diversity in New World, one endemic).
EUBLEPHARIDAE ‣ (6/)48 spp. (Repfocus/Eublepharidae), mainly Africa and Asia, but Coleonyx (9) from SW USA to Costa Rica.
SPHAERODACTYLIDAE ‣ (12/)239 spp. from C Mexico to E Brazil and West Indies, as well as in southern Europe, North Africa, the Middle East, and into Central Asia (Repfocus/Sphaerodactylidae), (5/)16 in Brazil (8 endemic). Only two another genera occur in New World: Aristelliger (9, Caribbean, 1 in Belize) and Sphaerodactylus (108, C Mexico to Ecuador, Venezuela and Caribbean, 7 in South America). (3/)6 spp. in Mexico. Highest diversity worldwide in Venezuela (28) and República Dominicana (25); Brazil has the 7th (Repfocus/Ranking).
PHYLLODACTYLIDAE ‣ (10/)171 spp., New World, North Africa, Europe and the Middle East (Repfocus/Phyllodactylidae). (4/)12 spp. in Brazil (7 endemic, Gymnodactylus a endemic genus). Three New World genera do not occur in Brazil: Garthia (2, endemic to Chile), Phyllodactylus (69, California to Chile in western flank of New World) and Tarentola (34, Africa and Europe, one in Caribbean). Mexico has (2/)19 spp. (15 endemic). Highest diversities are Mexico (21), Peru (17), Ecuador (14) and Brazil (12), by Repfocus/Ranking.
GEKKONIDAE ‣ (60/)1,734 spp. worldwide (Repfocus/Gekkonidae). Only two genera occur naturally in New World, from S USA to Uruguay and Caribbean: Lygodactylus (103, 101 in Africa and two in C Brazil, one of them up to Bolivia and Paraguay) and Hemidactylus (207). Brazil has the greatest diversity in the New World, but only the 92nd in the world (Repfocus/Ranking). Only 4 spp. of Hemidactylus occur in New World, H. agrius Vanzolini (NE Brazil), H. brasilianus Carranza & Arnold 2006 (NE & C Brazil), H. haitianus Powell, Henderson, Adler & Dundee 1996 (Cuba, República Dominicana, Haiti) and H. palaichthus Kluge (N Brazil, Colombia, Guyana, Saint Lucia, Surinam, Trinidad & Tobago and Venezuela), being Gekkonidae not recorded in natively in North and America Central.
SCINCOMORPHA
Four families, two only Old World: Gerrhosauridae (7/38, subsaarian Africa and Madagascar) and Cordylidae (10/69, Uganda to South Africa). Only one family in South America.
XANTHUSIIDAE ‣ (3/)38 spp. (Repfocus/Xanthusiidae) in Xantusia (14, SW USA and Baja California), Cricosaura (1, endemic to SE Cuba) and Lepidophyma (23, S Mexico to N Panama).
SCINCIDAE ‣ (168/)1,817 spp. worldwide (Repfocus/Scincidae). Mexico has (4/)37 spp. in Marisora in Mabuyinae, Mesoscincus and Plestiodon in Scincinae, and Scincella in Sphenomorphinae. All South American members of this family are Mabuyinae (Pereira & Schrago, PeerJ, 2017), subfamily with 25 genera, being nine only in Old World, Alinea, Capitellum, Mabuya and Spondylurus endemic to Caribbean islands, Maracaiba only in Venezuela and Colombia, Morisora only in Mexico, America Central and Caribbean, and all remaining 10 genera present in Brazil (16, two endemic genera, Brasiliscincus and Psychosaura). In Brazil occur Trachylepis, genus widely distributed and restricted of Africa except Trachylepis atlantica Bauer 2003, endemic to Fernando de Noronha Island off NE Brazil. In New World the two largest diversities are Mexico (34, 19th in World) and Brazil (15, 42nd in World), by Repfocus/Ranking.
LACERTIFORMATA
Only one family, Lacertidae (43/394, Repfocus/Lacertidae), a large lineage not recorded in New World, from Scandinavia and British islands to South Africa, Madagascar, up to Siberia, Japan, Java and Borneo, however not recorded in Philippines or Australasia.
AMPHISBAENIA
Six families, two only in Old World: Blanidae (1/7, Morocco, Portugal, Spain, Greece to Syria) and Trogonophidae (4/8, North Africa, the Horn of Africa, the Arabian Peninsula, and western Iran). All South American genera occur in Brazil (Repfocus/Amphisbaenia).
AMPHISBAENIDAE ‣ (16/)196 legless lizards, nine genera in Africa and adjacent regions and seven in South America (Repfocus/Amphisbaenidae): Amphisbaena (89, Panama to S Argentina and West Indies, 50 in Brazil), Anops (3, C Brazil, and S Brazil, N Argentina, Uruguay and S Bolivia), Aulura (1, endemic to N Brazil), Bronia (4, endemic to N & C Brazil), Cercolophia (6, Brazil to E Bolivia, Paraguay and N Argentina), Leposternon (11, Brazil and adjacent Bolivia, Paraguay and NE Argentina) and Mesobaena (2, one in Colombia and Venezuela, another in N Brazil). Vidal et al. (Biology Letters, 2008) provides a phylogeny of Amphisbaenidae.
BIPEDIDAE ‣ only one genus, Bipes, with three spp., all endemic to Mexico, one in S Baja California Sur, and two in C Mexico (possibly reports in SW USA, by Rorabaugh in US Fish and Wildlife Service, 2024).
CADEIDAE ‣ two spp. in Cadea, endemic to W Cuba and adjacent islands.
RHINEURIDAE ‣ only one sp., Rhineura floridana Cope, 1861, endemic to N & C Florida and adjacent Georgia, SE USA.
ANGUIMORPHA
Three families not recorded in New World: Shinisauridae (1/1, SE China and N Vietnam), Lanthanotidae (1/1, N Borneo) and Varanidae (1/89, Turkyie and Caspain region to S Australia and Pacific islands). Two genera in South American, both in Brazil. Mexico has the highest diversity in New World.
ANGUIDAE ‣ (22/)174 spp. worldwide (Repfocus/Anguidae), with 16 genera in New World (7 endemic to Caribbean), only three in South America, two in Brazil (Advenus only in Panama and Colombia): Diploglossus and Ophiodes. Mexico has the largest diversity worldwide (6/52), Brazil is the 9th (2/5, two endemic), by Repfocus/Ranking.
ANNIELLIDAE ‣ (1/)6 spp. from C California to NW Mexico.
HELODERMATIDAE ‣ 5 spp. in Heloderma, 3 spp. from Pacific coast of Mexico, one up to SW Nevada in USA, and one endemic to SE Guatemala.
XENOSAURIDAE ‣ 14 spp. in Xenosaurus, from NE Mexico to C Guatemala.
TEIFORMATA
All families occur in South America and in Brazil. 74 genera, 48 in Brazil (24 endemic).
ALOPOGLOSSIDAE ‣ (1/)32 spp., Costa Rica to Bolivia and Brazil (Repfocus/Alopoglossidae), mainly in Colombia (20), Ecuador (9) and Brazil (5), by Repfocus/Ranking.
GYMNOPHTALMIDAE ‣ (50/)302 spp. from Mexico to C South America (Repfocus/Gymnophthalmidae), (37/)99 spp. in Brazil (largest diversity worldwide, Repfocus/Ranking), 23 endemic. There are more endemic genera in Brazil than outside of it. Only one species occurs in Mexico, Gymnophthalmus speciosus Hallowell, 1861. Genera not recorded in Brazil: Centrosaura (Costa Rica and Panama), Anadia (Costa Rica to Ecuador and Guyana), Echinosaura (Panam to Ecuador), Pholidobolus (Panama to Peru and Venezuela), Kaieteurosaurus, Pantepuisaurus, Rheosaurus, Yanomamia (Guyana), Adercosaurus (Venezuela), Riama (Venezuela to Ecuador), Euspondylus (Venezuela to Peru), Magdalenasaura (Colombia), Andinosaura, Gelanesaurus (Colombia and Ecuador), Macropholidus, Selvasaura (Ecuador and Peru), Dendrosauridion, Petracola and Wilsonosaura (Peru).
In vertebrates, true parthenogenesis is found only in Squamata and (mostly) originates via interspecific hybridization after secondary contact (Brunes et al., Molecular Phylogenetics and Evolution, 2019). Leposoma percarinatum Müller, a parthenogenetic species from northern South America, has 66 chromosomes, one of the few known examples of a triploid genome in vertebrates (Catalogue of Organisms).
TEIIDAE ‣ (18/)179 spp. in New World (Repfocus/Teiidae), (7/)42 in Brazil. 7 genera do not occur in Brazil: Aspidoscelis (46, Mexico and USA), Aurivela (2, Argentina), Dicrodon (3, Peru to Ecuador), Holcosus (18, Mexico to Ecuador), Medopheos (1, Peru and Ecuador), Pholidoscelis (20, Caribbean) and Callopistes (4, Ecuador to Chile). Brazil has the largest diversity of genera (Glaucomastix endemic) and 2nd in species (42, Repfocus/Ranking). Mexico has the largest diversity of species (54) within two genera: Aspidoscelis and Holcosus.
IGUANIA
Three families only in Old World: Chamaeleonidae (12/235, mainly in Madagascar), Opluridae (2/8, Madagascar and Gran Comores) and Agamidae (62/634). Brazil has 7 families, and Mexico has 5. South America has 28 genera, 17 of them in Brazil (3 endemic).
PHRYNOSOMATIDAE ‣ (9/)179 spp. from SW Canada to Panama, 121 in Sceloporus.
CROTAPHYTIDAE ‣ (2/)12 spp. from SW USA to NW Mexico (Wikipedia).
LEIOCEPHALIDAE ‣ only Leiocephalus (24), all from West Indies (Repfocus/Leiocephalidae).
CORYTOPHANIDAE ‣ (3/)11 spp., C Mexico to Ecuador and Venezuela, unknown in Brazil — Basiliscus and Corytophanes occur in South America.
IGUANIDAE ‣ (9/)47 spp. from SW USA to S Brazil, Caribbean, disjunct in Fiji (Repfocus/Iguanidae), highly diverse in Mexico (5/19), America Central and West Indies. 4 genera occur in South America: Amblyrhynchus and Conolophus, endemic to Galapagos in Ecuador, Ctenosaura (15, NW Mexico to Panama, one up to NW Colombia) and Iguana (4, two in Lesser Antilles, one from Mexico to Costa Rica, and one from Costa Rica to South America and Lesse Antilles). I. iguana Van Denburgh 1898 is one of the most comoon lizards in urban areas in N & NE Brazil and unique member of this family in vast area in South America.
ANOLIDAE ‣ (8/)454 spp. from New World (Repfocus/Anolidae), SE USA to SE Brazil. Six genera outside South America, mainly Caribbean: Anolis (45, SE USA, Mexico to Honduras and Caribbean), Audantia (14, Hispaniola), Chamaelinorops (8, Hispaniola), Ctenonotus (43, Caribbean), Xiphosurus (14, Cuba, Hispaniola and Puerto Rico), Deiroptyx (32, Cuba, Hispaniola and Puerto Rico). Two occur in South America: Dactyloa (98, Costa Rica to N South America, disjunct E Brazil, and Caribbean) and Norops (201, NW Mexico to S Brazil and Caribbean). Highest diversities in Colombia (77), Cuba (65) and Mexico (55), in Repfocus/Ranking. Brazil has only 18 spp. (14th position, 7 in Dactyloa and 11 Norops).
POLYCHROTIDAE ‣ 8 spp. in a single genus, Polychrus, from Nicaragua to NE Argentina and SE Brazil (Repfocus/Polychrotidae). Highest diversities are Ecuador (5), Peru (5), Bolivia (3) and Brazil (3), by Repfocus/Ranking).
HOPLOCERCIDAE ‣ (3/)22 spp. in northern South America (Repfocus/Hoplocercidae), in Enyalioides (18, Colombia to Peru and NW Brazil), Hoplocercus (1, C Brazil and e Bolivia) and Morunasaurus (3, Panama to N Peru). Highest diversities are Peru (12), Ecuador (11), Colombia (8) and Brazil (3), by Repfocus/Ranking.
TROPIDURIDAE ‣ (8/)148 spp. in tropical and subtropical South America (Repfocus/Tropiduridae), all genera in Brazil (two endemic) except Microlophus (24, Galapagos and Ecuador to Chile). Highest diversities in Peru (57) and Brazil (42), by Repfocus/Ranking.
LEIOSAURIDAE ‣ (6/)36 spp. from Brazil and adjacent Bolivia, Paraguay, Argentina and Uruguay (unknown in Colombia, Repfocus/Leiosauridae). 4 genera in Brazil (two endemic) and two outside: Diplolaemus and Pristidactylus, both from Argentina and Chile. Highest diversities in Argentina (17) and Brazil (15).
LIOLAEMIDAE ‣ (3/)362 spp. from Peru to Patagonia and S Brazil (Repfocus/Liolaemidae), including the largest Squamata genus, Liolaemus (306), highly centered in Argentina. In Brazil occur 3 spp., 2 endemic (Intreasures), slightly disjuncts from coast of Rio de Janeiro state to S Rio Grande do Sul state. Phymaturus (56, Argentina and Chile) and Ctenoblepharys (1, endemic to coasts of Peru) do not occur in Brazil.
SERPENTES
(27:565/)4,264 spp. worldwide (Repfocus/Serpentes). Brazil has the second largest diversity of snakes worldwide (420), after Mexico (438), by Repfocus/Rankings, 20260217). 8 genera of non-Colubridae snakes occur in South America but non in Brazil.
17 families do not occur in New World: Gerhophilidae (2/30, non venomous, India, Indonesia, Papua New Guinea, Philippines, Sri Lanka, Thailand), Xenophidiidae (1/2, non venomous, Peninsular Malaysia and Borneo), Uropeltidae (7/73, non venomous, India and Sri Lanka), Anomochilidae (1/3, non venomous, Peninsula Malaysia, Sumatera and Borneo), Cylindrophiidae (1/15, non venomous, India to Sulawesi), Xenopeltidae (1/3, non venomous, India to Sulawesi), Pythonidae (10/40, non venomous, tropical Africa, India to S Australia), Acrochordidae (1/3, aquatic, non venomous, India to NE Australia and Philippines), Xenodermidae (6/37, non venomous, S India to Java and Japan), Pareidae (4/46, non venomous, E India and S China to Sulawesi), Homalopsidae (29/60, non solenoglyphous venomous, Pakistan, Sri Lanka, E India to NE Australia) and Lamprophiidae (62/337, sometimes venomous, S Europe, Africa, C, S & SE Asia).
10 families in New World, all in Brazil except Loxocemidae. The main deficiencies of Brazil regarding snakes in the New World are the absence of Loxocemidae and the lack of primacy in species richness in Leptotyphlopidae (after Peru) and Typhlopidae (after Cuba and Haiti), as well as in number of genera in Boidae (after Colombia), Anomaleptidae (after Colombia and Ecuador), and Tropidophiidae (after Cuba and Ecuador). Additionally, there is the explosive radiation of Viperidae in Mexico (41 more species, 6 more genera, 2 endemic genera) and Colubridae (including the absence of Sibynophiinae, Carpophiinae, and Natricinae), as well as the unfortunate absence of sea snakes in the country.
LEPTOTYPHLOPIDAE ‣ no venomous. SW USA to C Argentina, Caribbean, Africa to India. 7 genera in New World, Tetracheilostoma in Lesser Antilles, Mitophis in Hispaniola, Rena from USA to Mexico, and 4 in Brazil (largest diversity in New World along French Guiana). Brazil has the 2nd diversity in World (18, after Peru/19, Repfocus/Leptotyphlopidae).
TYPHLOPIDAE ‣ no venomous. (19/)280 spp., pantropical, in New World from S Mexico to NE Argentina and Caribbean. Brazil has 21st diversity in World (1/7, Repfocus/Typhlopidae), and 3rd in New World after Cuba (12) and Haiti (10). Cuba and Bahamas has two genera each.
ANOMALEPTIDAE ‣ no venomous. (4/)23 spp., Costa Rica to Paraguay and Brazil. Brazil has (2/)9 spp., largest diversity of species worldwide (Repfocus/Anomaleptidae), however Colombia and Ecuador has three genera each (including Anomalepis and Helminthophis). Unknown in Mexico.
ANILIDAE ‣ no venomous. A single sp., Anilius scytale Oken 1816, from Caribbean and Choco region up to C and NE Brazil in Ceará state (Repfocus/MAP). Unknown in Mexico.
TROPIDOPHIDAE ‣ no venomous. (2/)37 spp., in Trachyboa (3, Panama to Ecuador) and Tropidophis (35, 28 in Caribbean, 4 from Ecuador to Peru, and three endemic to Brazil, 3rd diversity worldwide after Cuba and Ecuador, Repfocus/Tropidophiidae). Unknown in Mexico.
BOIDAE ‣ no venomous. (14/)67 spp., nine genera in New World, 4 in Brazil, Charina (2, USA and Canada), Chilabothrus (14, insular Caribbean), Exiliboa (1, Mexico), Lichanura (USA to Mexico) and Ungaliophis (1, Mexico to Colombia). Brazil has the largest diversity of species worldwide (4/12, SEE). 5 genera in Colombia.
Eunectes akayima Rivas et al., 2024, for northern South America is described in Rivas, JA et aL. (Diversity, 2024), but non valided in Repforcus/Eunectes in Feb 23, 2026.
LOXOCEMIDAE ‣ no venomous. Only one sp., Loxocemus bicolor Cope, 1861, in Pacific coast from C Mexico to NW Costa Rica.
ELAPIDAE ‣ (57/)410 spp. worldwide (Repfocus/Elapidae), including terrestrial and marine forms, many very venomous. 4 genera in New World: Hydrophis (1, Indo-Pacific), Leptomicrurus (4, northern South America, Amazonas, Pará, Roraima and Amapá states in Brazil), Micruroides (1, W North America from Arizona to C Mexico) and Micrurus (86, E USA to C Argentina, excludes Caribbean). Among terrestrial species, Brazil has the second diversity in World (37, Repfocus/Ranking, after Australia). Among all New World countries, Brazil has the largest diversity, ahead Colombia (31) and Ecuador (21).

UNBRAZILIAN SNAKE GENERA IN SOUTH AMERICA EXCEPT COLUBRIDAE AND VIPERIDAE
Sea snakes are now considered part of the family Elapidae. They were previously regarded as one or two separate families, Hydrophiidae and (or including) the sea kraits, Laticaudidae (or Laticaudinae). Sea snakes live all their lives in the sea and are absolutely unknown in the Atlantic. In the New World there is only one species, exactly the wider distributed, Hydrophis platurus L., 1766, having been collected from California to the extreme NW Peru (Quiñonez et al., CheckList, 2014). For sea snakes and world diversity, see Elfes et al. (Herpetological Conservation and Biology, 2013). About why there are no sea snakes in the Atlantic, see Lillywhite et al. (BioScience, 2018).
VIPERIDAE ‣ venomous. All vipers in New World are Crotaliinae, with 12 genera in region, nine in Mexico, the center of diversity of family (72), including two endemic genera. Brazil has 3rd world diversity, with (4/)31 spp. (RepfocusRanking), after Mexico and China.
Six New World genera do not occur in South America: Agkistrodon and Sistrurus from E U.S.A to Mexico, Mixcoatlus and Ophryacus endemic to Mexico; and Metlapilcoatlus and Cerrophidion from Mexico to America Central. Four genera reaches to Mexico to South America: Bothrops (51, 2 in Caribbean, one widely in South America up to Mexico, and remaining 48 only South America, with 27 in Brazil, 13 endemic, and endemic also in Argentina, Colombia, Peru, Bolivia, Venezuela and Ecuador), Crotalus (51, 49 from Canada to Panama, one endemic to Venezuela, and the widely distributed South American restricted C. durissus L., 1758), Porthidium (9, Mexico southward through America Central to Ecuador in the Pacific lowlands, northern Venezuela in the Atlantic lowlands, 3 in South America, not recorded in Brazil) and Bothriechis (19, Mexico, through America Central to Colombia, western Venezuela, Ecuador and northern Peru, only one in South America, unknown in Brazil).
Three genera do not occur in Mexico: Atropoides (1, Costa Rica and Panama), Bothrocophias (8, Colombia to Brazil. 2 in Brazil, endemic in Ecuador and Colombia) and Lachesis (4, Costa Rica to Bolivia and C Brazil, 1 in Brazil, 3 in South America).
SOUTH AMERICAN RANGE OF UNBRAZILIAN VIPERIDAE GENERA IN SOUTH AMERICA
COLUBRIDAE ‣ (274/)2,224 spp. worldwide (RepfocusColubrudae), 103 genera in Old World and 171 in New World, being 62 only in South America, 71 only North America to Panama and Caribbean, and 32 in both areas. Here we follow Wikipedia with regard to families, unifying in the form of subfamilies under Columbridae several groups that the other master reference for snakes, Wallach et al. (BOOK, 2014, parameter used in this discussion) segregates as independents; however, here we take Carpophiidae as subfamily, which Wikipedia does not highlight. Thus, five subfamilies occur in New World. Brazil has 302 spp., second diversity worldwide, after Mexico with 323 (Repfocus).
SIBYNOPHIINAE
Two genera, Sibynophis from SE Asia and Scaphiodontophis (2) in New World, from E Mexico to NW Colombia, ovelapping species only in Honduras and Nicaragua.
COLUBRINAE
126 genera, 52 genera in New World, mainly in USA (13, Cemophora endemic), Mexico (33, Conopsis, Geagras, Pseudoficimia and Sympholis endemic). Brazil has 12 genera: Chironius, Dendrophidion, Drymarchon, Drymobius, Drymoluber, Leptophis, Mastigodryas, Oxybelis, Palusophis, Phrynonax, Simophis, Spilotes and Tantilla), none endemic. In South America also occur Lampropeltis (Canada to Venezuela and Ecuador), Masticophis (USA to Colombia and Venezuela), Rhinobothryum (Nicaragua to Venezuela to Ecuador) and Stenorrhina (Mexico to Ecuador and Venezuela).
CARPOPHIINAE
(4/)10 spp. in southern Canada to center Mexico: Carphophis (E USA), Contia (W USA and sw Canada), Diadophis (SE Canada to C Mexico and W USA), and Farancia (E & SE USA).
DIPSADINAE
103 genera, Thermophis endemic to Central Asia and all 102 remaining restricted of New World. 14 genera endemic to Caribe, 28 in Mexico (7 endemic, Cenaspis, Chersodromus, Cryophis, Manolepis, Pseudoleptodeira, Rhadinophanes, Tantalophis), 51 in Brazil (12 endemic, Amnesteophis, Caaeteboia, Cercophis, Coronelaps, Ditaxodon, Elapomorphus, Gomesophis, Lioheterophis, Ptychophis, Rodriguesophis, Sordellina and Tropidodryas), 9 from USA/Mexico/America Central to Colombia/Venezuela/Ecuador (Amastridium, Diaphorolepis, Enuliophis, Enulius, Geophis, Nothopsis, Pliocercus, Rhadinaea and Tretanorhinus), three from Venezuela to Ecuador region (Emmochliophis, Plesiodipsas, Saphenophis), one endemic to Argentina (Pseudotomodon) and 7 in Peru but non in Brazil (Arcanumophis, Coniophanes, Incaspis, Pseudalsophis, Synophis, Tachymenis and Urotheca, the first endemic, three also in Colombia). Brazil has the largest diversity in species and genera (also in endemic genera) in this subfamily worldwide.
NATRICINAE
36 genera worldwide, nine genera in New World, six restricted for North America: Clonophis (E USA), Haldea (E & SE USA), Liodytes (SE USA), Regina (C & E USA), Tropidoclonion (C USA), Virginia (C, E & SE USA). Nerodia (SE Canada to S Mexico, along E USA), Storeria (S Canada to America Central up to C USA and WC Mexico), Thamnophis (N Canada to America Central, in over North America) extends to America Central.
■ endemic families in New World: Bipedidae (Amphisbaenia, 1/3, Mexico), Cadeidae (Amphisbaenia, 1/2, Cuba), Rhineuridae (Amphisbaenia, 1/1, USA).
34.12 TESTUDINES ‣ (14:96/)376 spp. worldwide (Repfocus/Turtles, 20260217, all data used here). Brazil has (8:19/)36 spp., the 3rd in global turtle diversity (Repfocus/Ranking, 20260217), with — the same number as Indonesia and Colombia — surpassed only by the USA (62) and Mexico (52). There are two major clades in Testudines: Cryptodira and Pleurodira.
An outstanding reference for this group, including images and distribution maps for nearly all species, is the Turtle Taxonomy Working Group (2017, Turtle Conservancy). All South American genera occur in Brazil except Chelydra. Of the 25 genera of Cryptodira found in the New World, only nine are present in South America. Brazil may hold the 7th highest diversity of endemic Testudines, with 7 endemic species in the families Chelidae and Emydidae (Intreasures), after the USA (40), Australia (29), Mexico (22), Ecuador (14), China (12), and Indonesia (11).
PLEURODIRA
A southern Hemisphere group, from South America, Africa, SW Arabian peninsula, Madagascar, Australia and New Guinea. In Pleurodira the neck is bent in the horizontal plane, drawing the head into a space in front of one of the front legs. A larger overhang of the carapace helps to protect the neck, which remains partially exposed after retraction. This differs from the method employed by Cryptodirans, which tucks its head and neck between its forelegs, within the shell. Three families, Pelomedusidae (2/9, Mauritania to South Africa, E Africa, Yemen and Madagascar) does not occur in South America.
CHELYDAE ‣ (15/)66 spp. in South America (7/22, Hydromedusinae and Chelinae) and Australia to New Guinea (8/44, Chelodininae and Pseudemydurinae). All South American species occur in Brazil (second diversity worldwide of this family with 20 spp., Repfocus), except Acanthochelys pallidipectoris Freiberg, 1945 from Argentina, Paraguay and Bolivia, and two Mesochlemys from Colombia and Venezuela, one endemic each.
PODOCNEMIDIDAE ‣ (3/)8 spp., Erymnochelys in Madagascar, and two in northern South America: Podocnemis (6, all occur in Colombia, 4 up to Brazil) and Peltocephalus (1, over northern South America).
CRYPTODIRA
11 families worldwide, Platysternidae (Wikipedia) and Carettochelyidae (Repfocus/Carettochelyidae) do not occurs is New World. In New World, Tryonichidae (only Apalone in New World), and Dermatemydidae (monotypic from S Mexico, Guatemala and Belize) occurs from Canada to America Central but non in South America. The seven remaining families worldwide occur in South America.
SOUTH AMERICAN RANGE OF ALL FIVE LAND CRYPTODIRA GENERA IN REGION
CHELYDRIDAE ‣ (2/)7 spp. in Macrochelys (1) endemic to USA, and Chelydra (3) from North America to Colombia and Ecuador in South America (only C. acutirostris Babcock, 1932 in continent).
DERMOCHELYIDAE ‣ only one sp., Dermochelys coriacea Blainville, 1816, widely in tropical and subtropical oceans. In Brazil D. coriacea nests regularly in northern coast of Espírito Santo state (Almeida et al., Endangered Species Research, 2011).
CHELONIDAE ‣ (5/)6, sea-turtles. All species breeds in Brazil except Lepidochelys kempii Baur, 1890, breeding only in Veracruz state of Mexico and some places in SE USA (NOAA), and Natator depressus Limpus, 1988 nesting only in northern Australia and southern New Guinea.
EMYDIDAE ‣ (12/)56 spp., highly centered in E North America in E USA and SE Canada (six exclusive genera in this zone), along Chrysemys, Pseudemys and Terrapene up to Mexico, Actinemys in Pacific coast from Oregon to NW Mexico, Emys (2) from Portugal to Kazakhstan and Iran, and Trachemys (17) with 13 spp. from USA to Panama, two in Colombia and Venezuela, one in S Brazil, Uruguay and Argentina, and one endemic to Maranhão and Piauí states in NE Brazil.
GEOEMYDIDAE ‣ (19/)77 spp. worldwide, only one genus occurs in New World: Rhinoclemmys (9): six species from America Central to Colombia, Venezuela and Ecuador zone (4 in Colombia), two in Mexico (one up to Costa Rica), and R. punctularia Daudin, 1801, from NE Venezuela to NE Brazil.
KINOSTERNIDAE ‣ (4/)34 spp., mainly from Mexico: Sternotherus (2) up to Canada, Claudius (2) and Staurotypus (6) up to America Central and Kinosternon (21) up to South America. Only three in South America, all in Colombia (one endemic), and only K. scorpioides in Brazil (N & NE). Mexico has 8 endemic species in this family, all in Kinosternon.
TESTUDINIDAE ‣ (17/)58 spp., only two genera in New World: Gopherus (5, Mexico and USA) and Chelinioides(17, 3 in mainland South America, two in Brazil, and 14 endemic to Galapagos Islands).
34.13 CROCODILIA ‣ (3:9/)28 spp. (Repfocus). All Crocodylia from New World occur in Brazil except the four Crocodylus (Crocodylidae) and one Alligator (Alligatoriidae); four of these species are mutually nationally disjuncts: C. rhombifer Cuvier, 1807 (endemic to W Cuba), C. moreletii Dumeril & Bibron, 1851 (SE Mexico, W Belize and N Guatemala), C. intermedius Graves, 1819 (E Colombia and W Venezuela) and Alligator mimississipiensis Gray, 1831 (endemic to SE USA). The fifth, C. acutus Cuvier, 1807, occurs sympatrycally with all four, plus range also in Peru, Ecuador, El Salvador to Panama, and other Greater Antilles (SEE). Brazil and Colombia has the largest diversities worldwide (Repfocus).
Crocodylus intermedius has records extremely close to Brazil, with the most notable one located only 200 km from our borders, in the northeast of the Guainia department up NW Amazonas state (Data Basin), but notably the species does not occurs in the national territory. Other maps in Morales-Betancourt, M.A. et al. (Biota Colombiana, 2014).
Recent studies suggest the existence of two likely Crocodylus species endemic to small islands off the coast of Yucatán, Mexico (Ávila-Cervantes et al., Molecular Phylogenetics and Evolution, 2025), and five Caiman species in E Brazil (Ornelas, IS et al, Molecular Phylogenetics and Evolution, 2026).
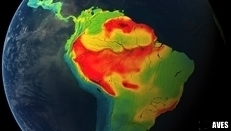
WORLD HOTSPOT OF AVIAN DIVERSITY (BM)
34.14 AVES ‣ (254:2,302/)11,887 spp. worldwide (Birds of the World | Catalogue of Life/Aves | Wikipedia). For details of diversity in South America, see South American Birds. For an overview of migratory birds in Brazil, see Somenzari, M. et al. (Papeis Avulsos de Zoologia, 2018).
46 living orders worldwide, 12 not breeds in New World: Struthioniformes (1:1/2), Apterygiformes (1:1/5), Casuariiformes (1:2/4), Mesitornithiformes (1:2/3), Pterocliformes (1:2/16), Otidiformes (1:12/16), Musophagiformes (1:5/23), Podargiformes (1:3/16), Aegotheliformes (1:1/10), Coliiformes (1:2/6), Leptosomiformes (1:1/1) and Bucerotiformes (4:19/75), and 34 breeds in New World: Rheiformes, Tinamiformes, Anseriformes, Galliformes, Columbiformes, Cuculiformes, Caprimulgiformes, Nyctibiiformes, Steatornithiformes, Apodiformes, Gruiformes, Charadriiformes, Phoenicopteriformes, Podicipediformes, Opisthocomiformes, Eurypygiformes, Phaethontiformes, Gaviiformes, Sphenisciformes, Procellariiformes, Ciconiiformes, Suliformes, Pelecaniformes, Cathartiformes, Accipitriformes, Strigiformes, Trogoniformes, Coraciiformes, Galbuliformes, Piciformes, Carimifomes, Falconiformes, Psittaciformes and Passeriformes. All orders that breed in the New World also breed in South America, except Gaviiformes.
Only two orders that breed in South America do not breed in Brazil: Sphenisciformes and Phoenicopteriformes. Two breeding orders in Brazil, Rheiformes and Cariamiformes, do not breed in Colombia. Cariamiformes, Opisthocomiformes, Steatornithiformes, and Rheiformes — all of which breed in Brazil — do not breed in either Mexico or the USA.
BRAZILIAN NUMBERS
For birds, there are many sources available to quantify avian diversity, with five being particularly relevant: CTFB (733/1969, SEE), Mongabay (1,813 spp. for Brazil in 2019, SEE), BirdLife Data Zone (1,816 spp. for Brazil as of May 1, 2025, SEE), WikiAves (1,963 spp. for Brazil as of May 1, 2025, SEE), and Birds of the World (1,818 spp, as of May 1, 2025, SEE). All these databases include vagrant and migratory species. Here, at Brazilian Metazoa, we choose to include only bird species that breed in Brazil. Therefore, our reference is a custom compilation, primarily based Brazilian Metazoa/South America Birds. In this compilation, we accept a total of (81:650/)1,712 breeding bird species in the national territory.
ENDEMISMS
In endemic species, Brazil has more than any other country in the Western Hemisphere and in the World is 3rd only to Indonesia and Australia: 263 spp. (Intreasures) or 257 (BirdLife). In endemic genera, Brazil has 25 against ✕ 8 Mexicans ✕ 3 Colombians ✕ 11 Peruvians. Excluding Passeriformes and Apodiformes, Brazil has 4 endemic genera (Psittaciformes-3 and 1 Piciformes-1), Colombia 1 (Psittaciformes), Peru 1 (Strigiforme) and Mexico has 3 (Galliformes, Psittaciformes and Trogoniformes).
USA
Among Passeriformes, including vagants, USA has 18 families unknown in Brazil (Laniidae, Paridae, Alaudidae, Remizidae, Aegithalidae, Sylviidae, Regulidae, Phylloscopidae, Sittidae, Certhiidae, Cinclidae, Icteriidae, Spindalidae, Calcariidae, Bombycillidae, Ptiliogonatidae, Acrocephalidae, and Peucedramidae), and Brazil has 12 amilies unknown in USA (Thamnophilidae, Pipridae, Cotingidae, Pipritidae, Melanopareiidae, Formicariidae, Furnariidae, Donacobiidae, Oxyruncidae, Conopophagidae, Rhinocryptidae and Mitronspingidae).
MEXICO
Except for Passeriformes, seven families breed in Mexico but not in Brazil.: Alcidae (4, Ptychoramphus aleuticus, Synthliboramphus scrippsi, S. hypoleucus and S. craveri), Phasianidae (2, Maleagris), Diomedeidae (Phoebastria immutabilis, breeding only off NW Mexico, and in Hawaii), Hydrobatidae (7 breeding species in Pacific coast of Mexico, 4 endemics), Pelecanidae (Pelecanus occidentalis), Pandionidae (1) and Phoenicoptridae (1). Except Passeriformes, Brazil includes 8 breeding families which do not beeds in Mexico: Rheidae, Opistochomidae, Cariamidae, Anhimidae, Psophiidae, Steatornitidae, Rostratulidae and Capitonidae. 12 breeding Passerifomes families in Mexico do not occur in Brazil: Alaudidae, Paridae, Aegithilidae, Remizidae, Ptiliogonatidae, Cinclidae, Regulidae, Sittidae, Rhodinocichlidae, Peucedramidae, Icteriidae and Spindalidae.
Among non-Passeriformes, Mexico has endemic species in 8 families in which Brazil do not have them: Odontophoridae, Apodidae, Momotidae, Falconidae and several seabirds: Hydrobatidae, Procellariidae, Laridae, Alcidae. Brazil has endemic species in 6 families in which Brazil do not have them: Anatidae, Accipitridae, Psophiidae, Galbulidae, Bucconidae, Capitonidae. Already on the South American neighbors, families in the same situation are found in Venezuela (Odontophoridae, Apodidae), Colombia (Odontophoridae, Podicipedidae), Ecuador (Spheniscidae, Diomedeidae, Procellariidae, Phalacrocoracidae, Laridae), Peru (Podicipedidae, Hydrobatidae), Argentina (Podicipedidae, Laridae) and Chile (Oceanitidae, Procellariidae, Laridae).
SOUTH AMERICA
Five South American breeding families are absolutely unknown in Brazil -
Sapayoidae (1/1, Panama to Ecuador, Wikipedia),
Cinclidae (1/5, one in North America, two in Eurasia, and two in South America, Wikipedia),
Rhodinocichlidae (1/1, Mexico to Colombia and Venezuela, in a very fragmented range, Wikipedia),
Pluvianellidae (1/1, Chile and Argentina, with recent records in Uruguay - Casteli et al., Ornithology Research, 2022) and
Semnornithidae (1/2, one species from Costa Rica and Panama, another from Colombia and Ecuador, Wikipedia).
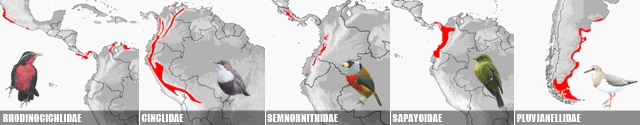
SOUTH AMERICAN RANGE OF ALL LAND BIRD FAMILIES UNKNOWN IN BRAZIL
It is worth mentioning Sapayoa, the only member of the New World of the Eurylaimides group (Moyle et al., American Museum Novitates, 2006), which is perhaps the most notable disjunction of birds in the South American continent. Bombycillidae is commonly reported as Colombian, however it´s a vagant/accidental/migrant up to NW Colombia, Venezuela and Ecuador (with only seven records in continent, Neotropical Bird Club), being excluded in our list.
NOTES FOR SOME GROUPS
GALIFORMES
In Cracidae, only a half of 10 genera in this family occur in Brazil. Outsiders are Pauxi and Aburria (Venezuela to Bolivia), Penelopina and Oreophasis (Mexico to Nicaragua), and Chamaepetes (Costa Rica to Peru).
STEATORNITHIDAE
A single species in this order, Steatornis caripensis Humboldt, 1817, an enigmatic bird whose occurrence in national territory is quite shrouded in imprecision. Wiki Aves reports only one occurrence in Brazil, with photos, in Manaus (Wiki Aves), ignoring a record made in 1998 at the Palmari reserve, of an individual killed and predated (Palmari), record reinforced by Tello et all. (Journal of Ornitology, 2008); both citations become insignificant in the face of Herrera (Boletin de la Sociedad Venezolana de Espeleologia, 2003), who record in quite extraordinary reports the presence of the species forming colonies just a few hundred meters from the border in Wei Tepui, northern Roraima state, Brazil.
SPHENISCIFORMES
Spheniscidae is the unique family in this order, with (6/)18 spp., six spp. breeds only in Australia and New Zealand, one only in southern Africa, five only Antartida and/or austral islands in Atlantic and Pacific Oceans, and six in South America (listing sites of breeding):
Aptenodytes patagonicus Miller, JF, 1778 (Kerguelen and Crozet, Prince Edward, Heard and McDonald, and Macquarie, South Georgia, Malvinas, southern Chile, South Sandwich and South Shetland, Bird Life).
Eudyptes chrysolophus Brandt, 1837 (the most common penguin, breeds Chile, Malvinas in Argentina, South Georgia and the South Sandwich, the South Orkney and South Shetland, Bouvet, Prince Edward and Marion, Crozet, Kerguelen, Heard and McDonald and very locally on the Antarctic Peninsula, Bird Life).
E. chrysochrome Forster, JR, 1781 (Malvinas islands off Argentina, Tierra del Fuego, Prince Edward and Marion, Crozet, Kerguelen, Heard and McDonald, The Animal Life).
Spheniscus magellanicus Forster 1781 (Atlantic and Pacific coasts of South America, in 67 sites in Argentina, at least 31 in Chile, and at least 100 in Malvinas Islands in Argentina, Bird Life).
S. humboldti Meyen 1834 (breeding in 49 sites from Isla Foca/5° 12´S in Peru down to Isla Guafo/43° 32´S in southern Chile, Bird Life).
S. mendiculus Sundevall 1871 (endemic to the Galápagos archipelago, Bird Life).
Non South American Spheniscidae:
Spheniscus demersus (breeds at 26 localities in N Namibia and E South Africa, SEE),
Megadyptes antipodes (SE coast of South Island in New Zealand, Stewart and outliers, Auckland group, and Campbell, SEE),
Eudyptes robustus (breeds on the Snares, 200 km south of New Zealand, SEE),
Eudyptes pachyrhynchus (W to SW coast of the South Island, New Zealand, SEE),
Eudyptes sclateri (breeds on the Antipodes and Bounty Islands in New Zealand, SEE),
Eudyptes schlegeli (breed only on Macquarie, Australia, SEE),
Eudyptula minor (not continuous from W Australia to New South Wales in Australia, Chatham to mainland New Zealand, SEE),
Eudyptes moseleyi (only southern islands, Tristan da Cunha, Gough, Amsterdam and St Paul, SEE),
Aptenodytes forsteri (54 colonies in Antartida, SEE),
Pygoscelis papua (Fish Islands on the Antarctic Peninsula to the Crozet Islands, SEE),
P. adeliae (entire Antarctic coast and at some of its nearby islands, SEE), and
P. antarcticus (Antarctica, South Sandwich, South Orkneys, South Shetland, South Georgia, Bouvet and Balleny, SEE).
PROCELLARIIFORMES
Only two species in this order breeds in Brazil, both in Procellariidae: Puffinus lherminieri Lesson, 1839, widely in topical areas, breeding only in Fernando de Noronha and Espírito Santo state in Brazil (Lopes, ACPA et al., Papéis Avulsos de Zoologia, 2014), and Pterodroma arminjoniana Giglioli & Salvadori, 1869, who breeds only in Trindade Island in Brazil, and in a small islet in Mauritius (IUCN Red List).
APODIFORMES
Brazil has only the 5rd diversity of Trochilidae, with 84 spp., after Colombia (164), Ecuador (135), Peru (133), and Venezuela (104).
PHOENICOPTERIFORMES
Phoenicopteridae, the sole family in this order, breeding in South America in Buenos Aires and Córdoba provinces in Argentina (Phoenicopterus chilensis, USP), coast of Venezuela and Galapagos islands in Ecuador (P. ruber, Birds Colombia, vagant in Brazil, very rare in N Colombia), and altiplane zone from Peru to Argentina and Chile (Phoenicoparrus, some vagant in Brazil). Mexico has one breeding species, in Yucatan region.
CATHARTIFORMES
Brazil has all the members of this order (with a single family, Cathartidae) except the two condors: Gymnogyps californianus
Shaw, 1797, the California Condor, from SW USA to NW Mexico; and Vultur gryphus L., 1758, Andean Condor, from NW Venezuela to Tierra del Fuego in W South America, one of the largest flying birds in the world, and is generally considered to be the largest bird of prey in the World.
PICIFORMES
Four of five genera of Ramphastidae occur in Brazil, the exception is Andigena (Wikipedia).
PSITTACIFORMES
There is a single parrot family in the New World, Psittacidae, with Brazil hosting 88 species. Ten New World genera within this family are not recorded from from Brazil: Conuropsis (USA), Rhynchopsitta (Mexico), Bolborhynchus (Mexico to Bolivia and Venezuela), Hapalopsittaca (Venezuela to Peru), Ognorhynchus (Colombia), Leptosittaca (Colombia to Peru), Thectocercus (Colombia to Argentina), Psilopsiagon (Peru to Argentina and Chile), Cyanoliseus (Argentina, Chile, and Uruguay), and Enicognathus (Chile and Argentina). Genera that are nearly restricted to Brazil include Pionopsitta (also found in Argentina and Paraguay) and Alipiopsitta (also in Bolivia). Brazil holds the highest diversity of Psittacidae genera in the New World — including three endemic genera — and also the greatest number of Psittaciformes species globally (Australia, by comparison, has only 56). In addition to the New World genera, two others in this family occur in Africa, while Australasian species belong to a separate family, Psittaculidae.
■ endemic families in New World: Nesospingidae (1/1, Puerto Rico) and Teretistridae (1/2, Cuba).
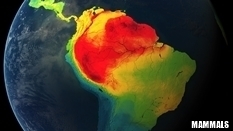
WORLD HOTSPOT OF MAMMAL DIVERSITY (BM)
35.15 MAMMALIA ‣ (153:1,360/)6,871 species worldwide according to the ASM website, organized in tabular format on the ASM Website/Table and by country on the ASM Website/Country. One of the best souces of visual mammals: the free pack of Handbook of the Mammals of the World.
Here, despite the existence of numerous references, we choose to consider Cetartiodactyla as divided into five distinct orders. This decision is based on the fact that it is simply too strange and taxonomically inconsistent to group Cetacea and Giraffidae within the same order. The relatively recent inclusion of Cetacea within Artiodactyla represents one of the most unusual outcomes that molecular phylogenetics has produced in recent years. What stands out most — and remains undeniably striking — is the profound morphological divergence between these groups, which makes their placement within a single order highly questionable. The idea of maintaining them together seems unnatural, so splitting Cetartiodactyla into separate orders appears to be the most coherent and scientifically sound alternative. However, it is important to note that there is currently no formal taxonomic restructuring in this direction. What exists, rather, is a growing recognition in the scientific literature that the group comprises five major clades, a division that has been strongly supported by multiple recent phylogenetic studies.
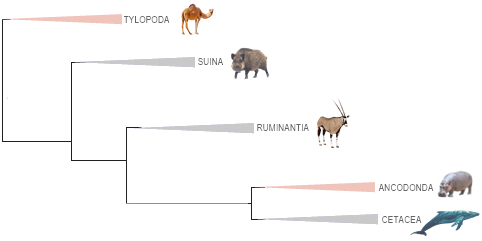
CETARTIODACTYLA PHYLOGENY ADAPTED FROM WIKIPEDIA (WIKIPEDIA)
Accordingly, of the 31 orders in our concept, 5 occur only in Africa to Madagascar or Middle East (Ancodonta, Afrosoricida, Tubulidentada, Macroscelida and Hyracoidea), 2 only in tropical Asia (Dermoptera, Scadentia), 2 widely from Africa to Asia (Proboscidea, Pholidota), 5 only from Australia to New Guinea and Australasia (Monotrema, Dasyuromorpha, Diprodontia, Notoryctemorphia, Peramelemorphia), and 17 in New World, all in South America, 13 in Brazil (Didelphimorphia, Sirenia, Pilosa, Cingulata, Rodentia, Lagomorpha, Primates, Cetacea, Ruminantia, Suina, Carnivora, Chiroptera and Perissodactyla) and 4 not recorded in (Eulipotyphla, Paucituberculata, Microbiotheria and Tylopoda).
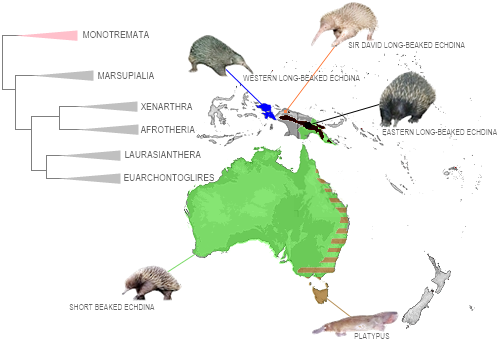
PHYLOGENY OF MAMMALS AND ALL EXTANT SPECIES OF BASAL MONOTREMATA, RESTRICTED TO EAST WALLACE LINE
BRAZIL
By a most recent checklist of mammals in country (Sociedade Brasileira de Mastozoologia/EXCEL, 2023: SBM/Brazil), Brazil has (49:248/)778 spp., in 13 orders: Didelphiomorpha (15/68), Pilosa (5/13), Cingulata (5/12), Chiroptera (68/182), Primates (20/130), Carnivora (25/36), Cetacea (28/49), Sirenia (1/2), Perissodactyla (1/1), Ruminantia (6/9), Suina (2/2), Rodentia (75/267) and Lagomorpha (1/4).
SOUTH AMERICA
South America has 343 genera of mammals in 64 families (2/3 bats or rodents). Brazil has the seconf largest diversity of mammals worldwide, after Indonesia (793), and followed by China (746), Mexico (585), Peru (582), Colombia (532), and Congo-Kinshasa (510), by Mammal Diversity Database/MAP. Colombia includes (51:213/)532 mammals (MDD/Colombia | Ramírez-Chaves et al., Mammology Notes, 2020). In all orders Brazil has more species than Colombia except Chiroptera, Perissodactyla and the not recorded in Paucituberculata and Eulypotyphla.
ENDEMISMS
Brazil has endemic genera in three orders (Chiroptera, Primates and Rodentia), Mexico in 5 (Chiroptera, Didelphiomorpha, Eulipotyphla, Lagomorpha, Rodentia) and USA in 2 (Chiroptera and Rodentia). Excepting Rodentia, endemic genera in South American countries occur in Brazil, Peru (Tomopeas: Molossidae), Ecuador (Cabreramops: Molossidae) and Argentina (Chlamyphorus: Chlamyphoridae; Chacodelphys and Lestodelphys: Didelphidae). Among endemic species, Brazil has the 3rd diversity worldwide (260, Intreasures), ahead Indonesia (342) and Australia (294), and ahead in China (230) and Madagascar (217). Brazil has endemic species of opossuns, river dolphins, armadillos, sloths, anteaters, bats, rabbits, fox, deers and monkeys.
ALBINISM IN ANIMALS
Several records of albinism in Brazilian and South American wildlife have been reported, including cases in Chiroptera (Leopoldo Ferreira de Oliveira Bernardi et al., Subterranean Biology, 2019), Coendou rufescens (Romero et al., Mammalia, 2017), Eira barbara (Aximoff & Rocha, Oecologia Australis, 2016), Lama guanicoe (Derlindati et al., The Southern Naturalist, 2013), and Proechimys spp. (Dalapicolla et al., Fapesp, 2020). Additionally, a high frequency of leucism has been documented in a population of Anoura geoffroyi in Minas Gerais, Brazil (Reis et al., Biota Neotropica, 2019).
SOUTH AMERICAN RANGE OF ALL LAND MAMMALIAN EXXOFAMILIES EXCEPT RODENTIA
SOUTH AMERICAN RANGE OF ALL RODENTIA EXXOFAMILIES
EXXOTAXA NOTES
Four South American orders do not occur in Brazil: Tylopoda (2/4, in Camelidae), Eulypotyphla (1/11, Cryptotis, Soricidae), Paucituberculata (3/7 spp. in Caenolestidae) and Microbiotheria (1/1, in Microbiotheriidae), with joined (5/)19 spp.
Among Brazilian orders, the seven exxofamilies are: Neobalaenidae (Caperea marginata Gray, 1846, found in temperate waters of the Southern Hemisphere, found in southern Chile and Argentina in South America), Ursidae (Tremarctos ornatus Cuvier, 1825 in east slopes of Andes from NW Venezuela to NW Argentina), Abrocomidae (2/10, Peru, Bolivia, Chile and Argentina), Octodontidae (7/14, Bolivia, Chile and Argentina), Chinchilidae (3/7, Ecuador to Chile and Argentina, SEE), Heteromyidae (5, all in Heteromys, from Venezuela to Ecuador) and Geomyidae (Orthogeomys dariensis Goldman, 1912, in NW Colombia).
ORDERS AND DIVERSITY IN NEW WORLD
PAUCITUBERCULATA
(3/)7 spp. in a single family, Caenolestidae (ASM Mammal Diversity), in Caenolestes (5, W Venezuela to N Peru), Lestoros inca O. Thomas, 1917 (SE Peru to W Bolivia, above 2,000m) and Rhyncholestes raphanurus Osgood, 1924 (S Chile and S Argentina).
MICROBIOTHERIA
Two spp. in Dromiciops (ASM Mammal Diversity), from S Chile and S Argentina.
DIDELPHIMORPHIA
(18/)131 spp. in Didelphidae (ASM Mammal Diversity), from USA to Uruguay. Brazil leads (fully or tied) in number of species in all genera of Didelphidae except Marmosa (Peru more 3, Colombia more 2), Lutreolina (Argentina and Bolivia more 1), Thylamys (Argentina and Bolivia more 1), and the three not recorded in: Tlacuatzin (5, Mexico), Chacodelphis (1, Argentina) and Lestodelphys (1, Argentina). For several Marsupialia in South America, see Louise & Myers (Field Museum, 2001).
SIRENIA
4 living species (ASM Mammal Diversity): Dugong dugon Müller, 1776 (Mozambique to New Caledonia and Taiwan, MPA), Trichechus manatus L., 1758 (Atlantic coasts from Virginia in USA to Bahia state in NE Brazil, Caribbean), T. senegalensis Link, 1795 (Mauritania to Angola, inland up to Mali and Niger) and T. inunguis Natterer, 1883 (Amazon System rivers, from Venezuela to Bolivia and N Brazil).
PILOSA
(4:5/)17 spp., living anteaters and sloths (ASM Mammal Diversity). All Pilosa occur in Brazil (including four endemic) except by Cyclops catellus (Bolivia), C. dorsalis (W Ecuador, Colombia, America Central, S Mexico), Tamandua mexicana (C Mexico to Venezuela and Peru), and Bradypus pygmaeus (endemic to Isla Escudo de Veraguas, a small island off the Caribbean coast of Panama).
CINGULATA
(9/)22 spp. (ASM Mammal Diversity) from S U.S.A to southern South America in two families, highly centered in Chaco region in South America and in Argentina, rarely in northern South America.
DASYPODIDAE ‣ 8 spp. in Dasypus. Brazil leads (5). Unbrazilian species: D. sabanicola (Colombia and Venezuela), D. pilosus (Peru) and D. mazzai (N Argentina).
CHLAMYPHORIDAE ‣ 14 spp., 7 in Brazil and 7 not recorded in: Cabassous centralis (Mexico to Colombia, Ecuador and Venezuela), C. chacoensis (Paraguay and Argentina), Chaetophractus vellerosus (Argentina, Bolivia, Chile and Paraguay), C. villosus (Argentina, Bolivia, Chile and Paraguay), Calyptophractus retusus (Bolivia, Paraguay and Argentina), Zaedyus pichiy (Argentina and Chile) and Chlamyphorus truncatus (Argentina). Calyptophractus, Chaetophractus, Zaedyus and Chlamyphorus do not occur in Brazil.
RODENTIA
(35:539/)2,754 spp. in five high lineages (ASM Mammal Diversity). 8 New World families do not occur in Brazil: five from North America up to Ecuador (Zapodidae, Aplodontiidae, Castoridae, Geomyidae and Heteromyidae) and three from Ecuador to Argentina and Chile (Chinchillidae, Abrocomidae and Octodontidae). Brazil and Mexico has simultaneously Erethizontidae (Brazil 12 ✕ 2 Mexico), Dasyproctidae (Brazil 9 ✕ 2 Mexico), Cuniculidae, Sciuridae (Brazil 8 ✕ 36 Mexico) and Cricetidae (Brazil 42/155 ✕ 24/139 Mexico). Mexico includes three unbrazilian families: Castoridae (1 in country), Geomydidae (19 in country) and Heteromyidae (41 in country), all Castorimorpha. Brazil includes 4 unmexican families: Caviidae (9), Ctenomyidae (1/8), Dinomyidae (1/1) and Echimyidae (17/64).
ANOMALUROMORPHA
Three families, Anomaluridae (2/6), Pedetidae (1/2) and Zenkerellidae (1/1), all from tropical Africa.
CASTORIMORPHA
Three families: Castoridae (1/2, Alaska to N Mexico, and France to C Russia and N Kazakhstan, MAP), Geomyidae (7/42, SE Canada to NW Colombia, MAP) and Heteromyidae (5/70, SE Canada to Ecuador and Venezuela, MAP).
CAVIIMORPHA
17 families, 7 only in Old World: Bathyergidae (5/26, sub-Saharan Africa), Ctenodactylidae (4/5, Africa), Petromuridae (1/1, SW Africa), Thryonomyidae (1/2, Africa), Heterocephalidae (1/1, Somalia, Ethiopia, Kenya), Hystricidae (3/11, Europe and the Levant, most of Africa, India, and SE Asia as far east as Flores), and Diatomyidae (1/1, Laos).
10 families in New World Erethizontidae (3/18), Chinchillidae (3/6), Dinomyidae (1/1), Caviidae (6/24), Dasyproctidae (2/15), Cuniculidae (1/2), Abrocomidae (2/10), Ctenomyidae (1/68), Echimyidae (35/101) and Octodontidae (7/15). Brazil includes 7 families, and Mexico has 5 (Erethizontidae-2, Dasyproctidae-2 and Cuniculidae-1). Chinchillidae (Ecuador to Uruguay, MAP), Abrocomidae (SE Peru, SW Bolivia, NW Argentina, NE Chile, MAP) and Octodontidae (SW Bolivia, E Chile, W Argentina, MAP) do not occur in Brazil.
Echimyidae includes 27 genera, 17 in Brazil (64, five genera endemic to Brazil, including the two bigger endemic genera of mammals for a any New World country, Phyllomys-14 and Trinomys-10) and 10 unknown in country: five in Caribbean islands (Capromys, Geocapromys, Mesocapromys, Mysateles, Plagiodontia), and five from Nicaragua to Peru and Venezuela: Diplomys (Panama to NW Ecuador), Hoplomys (1, Honduras to Ecuador), Olallamys (2, NW Venezuela and Colombia), Pattonomys (5, Venezuela to S Peru) and Santamartamys (1, N Colombia).
Cavia intermedia Cherem, Olimpio & Ximenez, 1999, known only from a small island in Santa Catarina state, Brazil, is world's rarest living mammal, maintaining a population of between 40 and 60 individuals (Click Petroleo e Gás, 2016).
MYOMOPRHA
Nine families, 7 only in Old World: Calomyscidae (1/12, Syria, Azerbaijan, Iran, Turkmenistan, Afghanistan, and Pakistan), Dipodidae (14/41), Sminthidae (1/19), Muridae (162/850, largest family of mammals), Nesomyidae (22/71, Africa and Madagascar), Platacanthomyidae (2/7, Asia) and Spalacidae (7/41, Africa and Eurasia).
Two families in New World: Zapodidae (3/11) and Cricetidae (156/859). Cricetidae includes five subfamilies, two of which are exclusive to the Old World (Arvicollinae and Cricetinae), and three restricted to the New World. Of the New World subfamilies, Tylominae (4/10) and Neotominae (16/140) are primarily centered in North America and have only two species in South America. Sigmodontinae, in contrast, is predominantly South American (all genera occur here), despite the type genus of the group being native to North America.
Only the tribes Sigmodontini, Ichthyomyini, and Oryzomyini extend beyond South America, and just 10 genera within these three tribes occur outside the continent (Sigmodon, Ichthyomys, Melanomys, Nephelomys, Oligoryzomys, Oryzomis, Sigmodontomys, Tanyuromys, Transandinomys, and Zygodontomys). In total, Cricetidae comprises about 106 genera in the Americas, 88 of which are found in South America — 76 of them exclusively (Paton, BOOK, 2015). Brazil alone is home to (44/)158 spp. Colombia includes (30/)82 spp. of Cricetidae (Ramírez-Chaves et al., Mammology Notes, 2020).
SUBFAMILY NEOTOMINAE
Four tribes and (16/)140 spp. from Canada to Ecuador (Lynx). Two genera in South America: Isthmomys (2, one in South America) and Reithrodontomys (20, Canada to Ecuador, only one in South America).
SUBFAMILY SIGMODONTINAE
11 subtribes and four genera incertae sedis. The circumscription adopted by Lynx (Banner), Patton (2015), and SBM/Brazil (2023) are largely congruent, differing primarily in the inclusion of a few species described after Patton (2015) and cited in SBM/Brazil (e.g., in Delomys, Juliomys, Rhagomys, Deltamys, Scapteromys, 8 new in Neacomys, all from Brazil), as well as in the allocation of several genera listed as Incertae Sedis by Patton (2015), but now assigned to specific tribes in Lynx (Banner) and SBM/Brazil (e.g., Juliomys placed in Wiedomyini), and a inclusion of Daptomys (recognized in SBM/Brazil) under Neustocomys in Patton (2015).
Sigmodontini - 14 spp. in Sigmodon (USA to northern South America, Lynx). One sp. in Brazil and 4 in South America.
Ichthyomyini - (5/)18 spp. (Lynx) in Anotomys (N Ecuador), Chibchanomys (2, Colombia to Peru), Neusticomys (6, northern South America, two in Brazil), Ichthyomys (4, Panama to Venezuela and Peru) and Rheomys. The last genus is not recognized in Paton (2015), and Brazilian list recognizes Daptomys as distinct from Neusticomys. (1/)2 spp. in Brazil.
Incertae sedis - (4/)8 spp. (Lynx) in Abrawayaomys (2, one in SE Brazil, one in NE Argentina and Paraguay), Chinchillula (1, S Peru, W Bolivia and N Chile), Delomys (3, SE Brazil and NE Argentina), and Neomicroxus (2, Venezuela to Colombia). (2/)4 spp. in Brazil.
Reithrodontini - only Reithrodon (3, Argentina, Chile, Uruguay, and S Brazil, Lynx, one sp. in last).
Oryzomyini - (29/)141 spp. in Aegialomys (2, Ecuador, Galapagos and Peru), Amphinectomys (1, NE Peru), Cerradomys (8, all in Brazil, also in Bolivia and Paraguay), Drymoreomys (1, SE Brazil), Euryoryzomys (1, N Peru), Eremoryzomys (6, widely in tropical South America, 5 in Brazil), Handleyomys (3, Colombia and Ecuador), Holochilus (6, widely in tropical South America, 5 in Brazil), Hylaeamys (7, widely in tropical South America, 5 in Brazil), Lundomys (1, S Brazil and Uruguay), Melanomys (6, Honduras to Peru and Venezuela), Microakodontomys (1, C Brazil), Microryzomys (2, Venezuela to Bolivia in Andean region), Mindomys (1, Ecuador), Neacomys (15, tropical South America, 13 in Brazil), Nectomys (5, tropical America, 3 in Brazil), Nephelomys (13, Costa Rica to Bolivia and Venezuela), Nesoryzomys (3, Galapagos), Oecomys (16, widely in tropical America, 15 in Brazil), Oligoryzomys (23, Mexico to Argentina and Chile, Caribbean, 10 in Brazil), Oreoryzomys (1, Ecuador to N Peru), Oryzomys (5, North America to Colombia and Venezuela), Pseudoryzomys (1, Peru to Argentina and Brazil), Scolomys (2, Ecuador, S Colombia, N Peru and S Brazil, one in latter), Sigmodontomys (1, Honduras to Ecuador and Venezuela), Sooretamys (1, S Brazil, Paraguay and NE Argentina), Tanyuromys (1, Costa Rica, Panama, and Ecuador), Transandinomys (2, Costa Rica to Peru), and Zygodontomys (2, Costa Rica to Brasil, one in latter country). (15/)71 spp. in Brazil.
Akodontini - (16/)83 spp. in Akodon (38, widely in tropical South America, 11 in Brazil), Bibimys (3, S Brazil, NE Argentina, Paraguay, 1 in Brazil), Blarinomys (1, E Brazil to NE Argentina), Brucepattersonius (7, Brazil to Argentina, 4 in former country), Castoria (1, S Brazil and NE Argentina), Deltamys (2, E Argentina, S Brazil and Uruguay, both in Brazil), Gyldenstolpia (2, N Argentina and C Brazil one each), Juscelinomys (2, C Brazil and E Bolivia one each), Kunsia (1, C Brazil to N Bolivia), Lenoxus (1, SE Peru to Bolivia), Necromys (7, widely in tropical South America, two in Brazil), Oxymycterus (15, Peru and Brazil to Argentina, 9 in Brazil), Podoxymys (1, Venezuelan side of Mount Roraima, unknwon in Brazilian side), Scapteromys (3, S Brazil, NE Argentina, Uruguay), Thalpomys (2, C Brazil), and Thaptomys (1, S Brazil, NE Argentina and Paraguay). (14/)40 spp. in Brazil.
Thomasomyini - (5/)74 spp. (Lynx) in Aepeomys (2, Venezuela), Chilomys (2, Venezuela to Ecuador), Rhagomys (3, one in Peru and Bolivia, another in SE Brazil, and one in Rondonia state in W Brazil), Rhipidomys (23, over tropical South America, 16 spp. in Brazil) and Thomasomys (44, tropical South America, NW Venezuela to W Bolivia). (2/)18 spp. in Brazil.
Andinomyini - (2/)3 spp. (Lynx) in Andinomys (1, S Peru to NW Argentina and NE Chile) and Punomys (1, highest elevational range of any species of mammals in the Neotropics, in SE Peru and W Bolivia).
Euneomyini - (3/)6 spp. (Lynx) in Euneomys (4, S Argentina and Chile), Irenomys (1, S Argentina and Chile) and Neotomys (1, C Peru to NW Argentina and Chile).
Wiedomyini - (4/)8 spp. (Lynx): Juliomys (4, SE Brazil, Paraguay and NE Argentina), Phaenomys (1, SE Brazil), Wiedomys (2, endemic to NE Brazil), and Wilfredomys (1, S Brazil and Uruguay).
Abrotrichini - (5/)17 spp. (Lynx) in Abrothrix (8, C Peru to Tierra del Fuego), Chelemys (2, WC Argentina to C Chile), Geoxus (1, S Chile and adjacent Argentina), Notiomys (1, S Argentina) and Pearsonomys (1, SW coast of Chile).
Phyllotini - (11/)59 spp. (Lynx) in Andalgalomys (3, SE Bolivia, N Argentina and Paraguay), Auliscomys (3, Peru to Chile and Argentina), Calassomys (1, Minas Gerais state in SE Brazil), Calomys (21, one sp. in Colombia and Venezuela, and remaining 20 from Brazil and Peru to Patagonia, 9 in Brazil), Eligmodontia (7, SE Peru to Chile and Argentina), Galenomys (1, SE Peru to NW Bolivia), Graomys (4, Argentina, Bolivia and Paraguay), Loxodontomys (1, C & S Chile and S Argentina), Phyllotis (15, Ecuador to Patagonia), Salinomys (1, N Argentina) and Tapecomys (2, C Bolivia to N Argentina). (2/)10 spp. in Brazil.
SUBFAMILY TYLOMYINAE
(4/)10 spp. in Nyctomys, Otonyctomys, Ototylomys and Tylomys (Lynx). Only one genus in South America, Tylomys, with a single species in continent, T. mirae Thomas, in Colombia and Ecuador.
SCIUROMORPHA
Three families: Aplodontiidae (1/1, Aplodontia rufa Rafinesque, 1817, from British Columbia to C California), Gliridae (9/30, Africa, Asia and Europe) and Sciuridae (64/321, worldwide). Brazil includes only (3/)8 spp., all Sciuridae, in Sciurus (6), Microsciurus (1) and Sciurillus (1).
LAGOMORPHA
(2:11/)113 living spp. wordlwide (ASM Mammal Diversity) in Ochotonidae (1/34) and Leporidae (10/77). Only three genera occur in New World: Lepus (35, 27 in Old World from Europe to Southern Africa, Siberia and SE Asia, and 8 in New World, from Canada and Greenland to Mexico), Romerolagus (R. diazi Ferrari-Pérez, 1893, C Mexico) and Sylvilagus (30, New World from Alaska to Patagonia).
Sylvilagus includes 30 spp., being 28 confined to a single zone: Canada/USA (6), U.S.A/Mexico (3), Mexico and America Central (8), Venezuela to Peru (9, one widely, one endemic to Venezuela, 5 endemic to Colombia and two endemic to Ecuador), Suriname (1) and Brazil (1, S. tapetillus O. Thomas, 1913). Two remaining species are widely distributed: S. brasiliensis L., 1758, widely in South America, and S. floridanus J. A. Allen, 1890, from Canada to Colombia and Venezuela.
PRIMATA
(84/)517 spp. in 16 families (ASM Mammal Diversity), 11 in Old World: Galagidae (6/19, sub-Sahara Africa), Lorisiidae (5/17, central Africa as well as in south and southeast Asia), Tarsiidae (3/14, Brunei, Indonesia, Malaysia and the Philippines), Cheirogaleidae (5/41, Madagascar), Daubentoniidae (1/1, Madagascar), Indriidae (3/19, Madagascar), Lemuridae (5/21, Madagascar), Lepilemuridae (1/25, Madagascar), Cercopithecidae (23/164, Old World), Hylobatidae (4/20, E Bangladesh to NE India to S China and Indonesia) and Hominidae (4/8, including domesticated Homo sapiens L., 1758, wild in Africa and SE Asia).
Five primate families are restricted to tropical America. A recent phylogenetic revision proposed the division of Saguinus into several new genera (Brcko et al., Mol. Phyl. and Evol., 2022), including a genus for the Oedipomidas (O. geoffroyi, O. oedipus, O. leucopus), which does not occur in Brazil and is distributed from Panama (O. geoffroyi) to Colombia (all, one endemic) — geographically disjunct from the core Saguinus s.s. Brazil, along with the DR Congo, Madagascar, and Indonesia, holds a leading position in global primate diversity (Estrada et al., PeerJ, 2018), with 130 recorded species, including 18 endemic ones (SBM/Brazil, 2023). The country hosts four endemic genera — exclusively found in the Atlantic Forest — and a larger near-endemic genus, Mico. Brazil harbors representatives of nearly all New World primate genera, with the exception of Oedipomidas, and the highest number of species for all genera, except for Oedipomidas, Aotus, and Cebus, which are less represented or not recorded in country.
PITHECIDAE
(6/)60 spp., 14 spp. do not occur in Brazil: Cheracebus aquinoi (Peru), C. medemi (Colombia), Pithecia milleri (Colombia, Ecuador and Peru), P. napensis (Ecuador and Peru), P. aequatorialis, P. isabela (these endemic to Peru), Plecturocebus caquetensis, P. ornatus (these endemic to Colombia), P. discolor (Colombia, Ecuador and Peru), P. urubambensis, P. oenanthe (these endemic to Peru), P. aureipalatii (Peru and Bolivia), P. olallae and P. modestus (these endemic to Bolivia).
ATELIDAE
(4/)23 spp., 8 are not recorded in Brazil: Alouatta pigra (Mexico/Guatemala/Belize), A. palliata (Mexico to Peru), A. arctoidea (Venezuela), A. sara (Peru/Bolivia), Ateles geoffroyi (Mexico to Panama), A. fusciceps (Panama/Colombia/Ecuador), A. hybridus (Colombia/Venezuela), and Lagothrix flavicauda (Peru).
CEBIDAE
(3/)19 spp., nine do not occur in Brazil: Cebus imitator (Honduras to Panama), C. capucinus (Panama to Ecuador), C. cesarae, C. malitiosus, C. versicolor (these endemic to Colombia), C. leucocephalus (Colombia/Venezuela), C. brunneus (Venezuela), C. aequatorialis (Ecuador/Peru), C. cuscinus (Peru/Bolivia), and Saimiri oerstedii (Costa Rica/Panama).
CALLITRICHIDAE
(7/)55 spp., of which 8 are not recorded in Brazil: Oedipomidas leucopus (Colombia), O. oedipus (Colombia), O. geoffroyi (Panama/Colombia), Saguinus lagonotus (Ecuador/Peru), S. tripartitus (Ecuador/Peru), S. illigeri (Peru), S. leucogenys (Peru) and S. nigrifrons (Peru).
AOTIDAE
A single genus, Aotus, and 11 spp., five in Brazil and six not recorded in: A. zonalis (Panama to Colombia), A. brumbacki (Colombia), A. jorgehernandezi (Colombia), A. griseimembra (Colombia/Venezuela), A. lemurinus (Colombia/Ecuador), and A. miconax (Peru).
EULIPOTYPHLA
(4:61/)589 spp. worldwide, sometimes called true insectivores (including fossorial and venomous mammals), with four families, three of these in New World (ASM Mammal Diversity).
SOLENODONTIDAE
Two species: Atopogale cubana W. C. H. Peters, 1861 from Cuba and Solenodon paradoxus J. F. von Brandt, 1833 from Hispaniola.
SORICIDAE
(28/)488 spp. worldwide, 220 in Crocidura, the largest mammal genus/SEE. Five genera in New World: Cryptotis (54, Canada to Peru and Venezuela, one in Canada, two in USA, 14 in Mexico and 15 in South America), Sorex, Notiosorex (4, N & C Mexico, two up to S USA, in California and Texas), Blarina (4, E North America from C Canada to Florida) and Megasorex (1, center Pacific coast of Mexico).
20 spp. of Cryptotis occur in South America (additional data in Therya, 2020 | Papéis Avulsos de Zoologia, 2014 | MAP in Venezuela): endemics in Venezula (4), Colombia (7), Ecuador (4) and Peru (1), plus C. perijensis, C. tamensis (both in Venezuela and Colombia), C. squamipes (Colombia and Ecuador), C. montivagus (Ecuador and Peru).
TALPIDAE
(19/)65 spp. worldwide, five genera in New World: Condilura cristata L., 1758 (star-nosed mole, SE Canada to Minnesota and Florida), Parascalops breweri Bachman, 1842 (SE Canada to Kentucky), Neurotrichus gibbsii Baird, 1858 (SW British Columbia to California), Scalopus aquaticus L., 1758 (E North America to SE Canada to extreme NE Mexico) and Scapanus (5, British Columbia to Baja California del Norte, USA and Mexico one endemic each, three in USA and adjacent countries, two up to Canada and one up to Mexico).
CHIROPTERA
(236/)1,474 spp. in 20 families (ASM Mammal Diversity). Yinpterochiroptera includes 7 families, all exclusive to the Old World: Pteropodidae (46/196), Hipposideridae (9/92), Rhinolophidae (1/114), Rhinonycteridae (4/9), Craseonycteridae (1/1), Megadermatidae (6/6) and Rhinopomatidae (1/6). Yangochiroptera includes 14 families, 5 only Old World: Miniopteridae (1/41), Cistugidae (1/2), Myzopodidae (1/2), Nycteridae (1/14) and Mystacinidae (1/2); and nine in New World: Phyllostomidae (61/230), Vespertilionidae (60/531), Molossidae (21/135), Noctilionidae (1/2), Natalidae (3/11), Thyropteridae (1/5), Furipteridae (2/2), Emballonuridae (14/55) and Mormoopidae (2/18).
IN SOUTH AMERICA
All families in South America occur in Brazil; remarkably, six of them has exactly a single outsider genus (mainly with restricted distribution). Phyllostomidae has four outsiders (and three endemic genera in Brazil).
Unbrazilian South American genera in Chiroptera are nine: Balantiopteryx (3, Emballonuridae, two spp. in South America, in Ecuador and Colombia), Chilonatalus (3, Natalidae, Caribbean, one in San Andrés islands of Colombia), Centurio (1, Phyllostomidae, one sp. from S USA to Colombia and N Venezuela), Leptonycteris (1, Phyllostomidae, Colombia, Venezuela and ABC island), Platalina (Phyllostomidae, one sp. from Peru and Chile), Echisthenes (Phyllostomidae, Bolivia, Colombia, Costa Rica, Ecuador, El Salvador, Guatemala, Honduras, Mexico, Panama, Peru, T.Tobago, and Venezuela; there is a single record from the United States state of Arizona), Mormoopsis (Mormoopidae, Belize, Colombia, Ecuador, El Salvador, Guatemala, Honduras, Mexico, Peru, T.Tobago, Venezuela, and Texas in the United States), Amorphochilus (Furipteridae, Ecuador to N Chile), Tomopeas (1, Vespertilionidae, endemic to Peru) and Mormopterus (2, Molossidae, Peru and Chile).
BRAZIL ✕ COLOMBIA
Compared to Brazil, Colombia has a global advantage of 33 spp. (Sociedade Brasileira de Mastozoologia, 2023 ✕ Ramírez-Chaves et al., Mammology Notes, 2020). By families, the positive advantages for Colombia (in parentheses) places only in three families: Mormoopidae (2), Natalidae (2) and Phyllostomidae (37).
In Phyllostomidae, Colombia has advantagae in 17 Brazilian genera: Sturnira (9), Anoura (7), Artibeus (4), Platyrrhinus (4), Choeroniscus, Gardnerycteris, Lonchorhina, Uroderma, Vampyressa and Vampyrodes two each, Carollia, Hsunycteris, Lonchophylla, Lichonycteris, Rhinophylla, Mimon and Vampyriscus one each. Colombian Centurio, Enchisthenes, Leptonycteris and Chiroderma do not occur in Brazil. Brazilian Dryadonycteris, Scleronycteris, Neonycteris, Xeronycteris, Chrotopterus, Ametrida and Pygoderma do not occur in Colombia.
BRAZIL ✕ MEXICO
For Chiroptera, we accepted for Mexico (8:66/)120 spp. for List of Mammals from Mexico (Wikipedia), and (9:68/)199 spp. for Brazil by Quintela et a. (2020, cited ahead). In Noctilionidae, the same two species occur in both countries. In Natalidae, both countries has a single Natalus species. In Thyropteridae, both countries has the same genus, however Brazil has 5 spp. and Mexico only one. Furipteridae, with a single species in Brazil, does not occurs in Mexico.
In Emballonuridae Brazil has (7/)17 spp. and Mexico has (6/)9 spp. In all Mexican genera in this family Brazil has equal/more species except in Balantiopteryx, genus not recorded in Brazil.
In Molossidae Brazil has (8/)29 spp. and Mexico has (6/)18. In all Mexican genera in this family Brazil has equal/more species except in Nyctimops, where Mexico has 3 against 2 in Brazil.
In diotypic Mormoopidae, both countries has 4 Pteronopus, however Mexico has a species of Mormoops.
In Phyllostomidae Mexico has (37/)55 spp., and Brazil has (43/)95 spp. Mexico includes Centurio, Enchisthenes, Macrotus, Choeronycteris, Hylonycteris, Leptonycteris and Musonycteris are not recorded in Brazil, and more species in Glossophaga, Mimon and Dermanura.
In Vespertilionidae Mexico has (12/)47 spp., and Brazil has only (5/)29. Mexico includes Lasionycteris, Antrozous, Baueru, Corynorhinus, Euderma, Idionycteris, Nycticeius and Pipistrellus are not recorded in Brazil, and more species in Myotis and Rhogeessa.
CARNIVORA
(129/)314 spp. in 16 families (ASM Mammal Diversity). Ailuridae, Eupleridae, Herpestidae, Nandiniidae, Prionodontidae, Viverridae and Hyaenidae do not occur in New World. For data from Carnivora, see Sepúveda & Marín (Mammalian Biology, 2022) and Carnívoros Brasileiros (ICMBIO).
FELIDAE ‣ all Canidae species in New World occur in Brazil except all species of Urocyon (2), Canis (4 in NW) and Vulpes (4 in NW, two boreal, two in center), and four species of Lycalopex. Lycalopex (SEE) occur in Argentina (3), Chile (3), Peru (3), Ecuador (2), Brazil (2), Bolivia (2), Uruguay (1).
CANIDAE ‣ all Felidae species in New World occur in Brazil except two Lynx and two Leopardus. All Felidae in New World occur in Argentina except both Lynx. For questionable Brazilian endemic Leopardus emilieae Thomas, 1914, see Nascimento e Feijó (Papéis Avulsos de Zoologia, 2017). For possibly new species recognized in the Leopardus colocola complex for S & E South America, see Nascimento et al. (Zoological Journal of the Linnean Society, 2021).
PROCYONIDAE ‣ 10 spp. of Procyonidae do not occur in Brazil, in Bassariscus (2, genus unknown in Brazil, USA to Panama), Nasuella (2, genus unknown in Brazil, Venezuela to Ecuador), Procyon (2 unknown in Brazil), Nasua (1 unknown in Brazil, exclusive Venezuela) and Bassaricyon (3 unknown in Brazil, from Honduras to Ecuador).
URSIDAE ‣ four Ursidae occur in New World: Ursus americanus Pallas, 1780 (black, from Alaska to C Mexico), U. arctos L., 1758 (brown, Europe to Canada and NW USA), U. maritimus Phipps, 1774 (white, in Artic shore in Russia, Alaska/USA, Canada and Greenland), and Tremarctos ornatus Cuvier, 1825 (black, from NW Venezuela to NW Argentina in Andean forests).
MUSTELIDAE ‣ (12/)20 spp. of Mustelidae occur in New World. Taxidea, Pekania, Mustela and Martes occur, in New World, only in Canada and USA. Enydra (1, northern Pacific from Japan to NW Mexico) and Gulo (1, Scandinavia, Russia, Mongolia, China, and from Alaska to NW USA) extends to Mexico. Lyncodon in monotypic and occurs in Argentina and Chile. Lontra includes 4 spp., one in North America, one Neotropical and two in SW & S South America. Neogale includes 4 spp., one in Amazon forest, one in Canada and USA, one from Canada to Bolivia, and one in Colombia. Brazil includes all species of Ptenoura, Galictis and Eira. All American Latina genera occur in Brazil except Taxidea, Enydra and Lycondon. For details among Colombia wessel, see (Therya, 2019). For details about the very rare Neogale africana Desmarest, 1818, native to Brazil, see Ramírez-Chavez et al. (Mammalian Species, 2014).
MEPHITIDAE ‣ (4/)15 spp., in Mydaus (2, Sumatra, Borneo and Palawan), Mephitis (2, C Canada to Nicaragua), Conepatus (4) and Spilogale (7, SW Canada to Costa Rica). The situation of Conepatus is confusing: C. chinga G.I.Molina, 1782 (Brazil and Peru southwards) and C. leuconotus H.Lichtenstein, 1832 (SW USA to Nicaragua) are fully recognized on Wikipedia (Wikipedia/Skunk) and ASM Mammal Diversity. C. semistriatus P. Boddaert, 1785 is recognized in both with a distribution from Mexico to N Peru and W Venezuela, but on Wikipedia, it is listed with a distribution in NE Brazil, which is not mentioned in ASM Mammal Diversity. Wikipedia recognizes C. humboldtii Gray, 1837 (S Argentina and Chile), and ASM Mammal Diversity recognizes C. amazonicus (H. Lichtenstein, 1838). Carnívoros Brasileiros (ICMBio) recognizes C. semistriatus. Here, we follow the distribution proposed on Wikipedia.
ODOBENIDAE ‣ Odobenus rosmarus L., 1758, the sole member of Odobenidae, occurs in NW Canada to Greenland, Beringia Zone in Alaska and NW Russia, and some points of northern coast of Russia (Wikipedia).
PHOCIDAE ‣ includes (14/)19 spp. in four geographycally consistent ranges, three mutually disjunct. SBM/Brazil (2023) includes four Phocidae species in country, all are errant and none of them breeds in Brazil, counted only anedoctal records: Lobodon carcinophaga Hombron & Jacquinot, 1842 (Rio de Janeiro to Rio Grande do Sul - Rodrigues, Steinwender & Ribeiro, Natureza Online, 2018), Mirounga leonina L., 1758 (in same area and records in Abrolhos, Bahia state - Salvatore et al., Boletim do Laboratório de Hidrobiologia, 2020, and Buloto & Mayorga, Natureza Online, 2015), Hydrurga leptonyx Blainville, 1820 (informal records, as OECO, 2019) and Leptonychotes wedellii Lesson, 1826 (Trindade island - Fainer et al., Polar Biology, 2018, the single record in Brazil and the northermost of ones).
Phocinae - (7/)10 spp. from northern Hemisphere: Halichoerus (1, NW USA, Canada, Iceland, N Europe up to Barents Sea), Histriophoca (1, Japan to Alaska), Pagophilus (1, Canada to Greenland up to N Russia), Pusa (3, Artic, Caspain and Baikal Lakes), Phoca (2, China to Mexico, Florida to Spain and Barents Sea), Erignathus (1, Japan to Alaska, Canada, NE USA, Greenland and some points in Norway and Russia) and Cystophora (1, NE USA, Canada, Greenland and Iceland).
Lobodontini - 4 monotypic genera from Antarctica: Leptonychotes, Hydrurga, Lobodon and Ommatophoca.
Monachini - two genera: Neomonachus (1, Hawaii) and Monachus (1, Mediterranean Sea and NW coast of Africa up to Mauritania).
Miroungini - one genera, the unique genus in both Hemispheres: Mirounga (2, one from Alaska to Mexico, another around in Antarctica and austral Oceans).
OTARIIDAE ‣ includes (5/)15 spp. worldwide in Otaria (1, Peru to Uruguay in coast), Neophoca (1, SW Australia), Callorhinus (1, Korea to Mexico in northern Pacific), Zalophus (3, Japan and Korea, Alaska to Mexico, and Galapagos) and Arctocephalus (8, three around South America from Galapagos to Uruguay, two in Austral Oceans, one from SW USA and NW Mexico, two around Australia and New Zealand, one of them also in Africa). SBM/Brazil (2023) includes four species of Otariidae in country (Sociedade Brasileira de Mastozoologia, 2023), however, as like Phocidae, none of them breeds in Brazil. Two of these are non breeding but have some population in some places of Rio Grande do Sul state: Arctocephalus australis Zimmermann, 1783 (Amorim, Tese, 2018), species with their large population (approx. 3/5) in Uruguay, and Otaris flavescens Shaw, 1800 (ICMBIO, 2011). The two remainig species are errant in country: A. tropicalis Gray, 1872 and A. gazella Peters, 1875 (ICMBIO, 2011).
The La Coronilla Islands (Verde Island and Coronilla Islet), in E coast of Uruguay, located only about 35 km from the southernmost tip of Brazil, constitute the closest pinniped breeding colony to Brazil (Szteren, Brazilian Journal of Oceanography, 2015).
TYLOPODA
(3/)7 spp. worldwide, all in Camelidae (ASM Mammal Diversity). Three species occur only in Old World: Camelus bactrianus L., 1758 (domesticated and feral, Asia), C. dromedarius L., 1758 (domesticated and feral, N Africa and Middle East) and C. ferus Przewalski, 1878 (wild, NW China and SW Mongolia).
Four occur in montane habitats in South America, all in Lama: L. glama L, 1758 (domesticated, 3.23 M worldwide, 3/5 in Bolivia, ICIMOD), L. guanicoe Müller, 1776 (wild, negligent populations in C Peru to SW Bolivia, and from S Bolivia to S Argentina along western mountains, and adjacent Chile, 600 K in Argentina, 220 K in Chile: MAP, ICIMOD, also in NW Paraguay, SEE), L. pacos L, 1758 (domesticated, 3.02 M worldwide, 4/5 in Peru, ICIMOD), and L. vicugna Molina, 1782 (wild, C Peru to NW Argentina and N Chile, MAP). Guanaco is the wild ancestor of the llama, while the vicuña is the wild ancestor of the alpaca (Kadwell, M., National Library of Medicine, 2001). For Camelidae from Peru, see FAO/2005.
SUINA
(9/)21 spp. worldwide in two families (ASM Mammal Diversity): Suidae (6/18) from the Old World and Tayassuidae in New World with three monotypic genera: Dicotyles tajacu L., 1758 from S USA to Uruguay; Tayassu pecari Link, 1795 from C Mexico to NE Argentina and S Brazil; and Parachoerus wagneri Rusconi, 1930, from SE Bolivia, W & C Paraguay and N Argentina, slightly closed for Brazilian border (MAP).
RUMINANTIA
(83/)223 spp. in six families (ASM Mammal Diversity), with three only in Old World: Tragulidae (3/10), Moschidae (1/7) and Giraffidae (2/5); and three in New World: Cervidae (22/57 worldwide), Bovidae (54/153 worldwide) and Antilocapridae (1/1).
BOVIDAE
(54/)153 spp. worldwide, with four genera and 5 spp. in New World: Oreamnos americanus Blainville, 1816 (Alaska to Colorado in W USA), Ovibos moschatus Zimmermann, 1780 (natively in Greenland and N Canada), Bos bison L., 1758 (former natively from Alaska to N Mexico), Ovis canadensis Shaw, 1804 (British Columbia to NW Mexico), and O. dalli Nelson, 1884 (Alaska to British Columbia).
For feral bubals in Brazil, see Carvalho et al. (Management of Biological Invasions, 2021) and a journalist reporting at Globoplay (SEE).
ANTILOCAPRIDAE
Sister of Giraffidae with a single species, Antilocapra americana Ord, 1815, known from SW Canada, W USA, N & NW Mexico.
CERVIDAE
(22/)57 spp. worldwide in five tribes. All 23 spp. from Cervidae in New World are Odoicoileini except the two massive, huge species native from Asia and North America: Cervus canadensis Erxleben, 1777 (Cervini), and Alces alces L. (Alceini), 1758, both the largest Cervidae in New World, the former up to N Mexico.
Odoicoleini (11/21) includes one genus in Rangiferini (Rangifer), two genera in Odocoileina: Odocoileus (3) and Mazama (7), and 8 in Bastocerina (all exclusives of South America). In Blastocerina, four genera are not recorded from in Brazil: Bisbalus (1, endemic to Venezuela), Pudella (2, Venezuela to C Peru), Pudu (1, Argentina and Chile) and Hippocamelus (2, Peru to NW Argentina); and four occur in Brazil: Passalites (1, northern South America up to Brazil and Bolivia), Blastocerus (1, Brazil, Peru, Bolivia, Argentina, Paraguay and Uruguay), Ozotocerus (1, Brazil, Bolivia, Argentina, Paraguay and Uruguay) and Sabulo (1, Brazil, Bolivia, Argentina, Paraguay and Uruguay).
Mazama includes 7 spp. (4 in Brazil, highest diversity), being M. americana Erxleben, 1777 (South America except Chile and Uruguay), M. jucunda O. Thomas, 1913 (SE Brazil, SEE), M. chunyi Hershkovitz, 1959 (Peru to Bolivia), M. nanus Hensel, 1872 (Brazil, Argentina and Paraguay), M. rufa Illiger, 1815 (Brazil, Argentina, Bolivia, Paraguay, SEE), M. rufina Pucheran, 1851 (Colombia, Ecuador and Peru) and M. temama Kerr, 1792 (Mexico to Colombia).
All Odocoleini species occur in South America except two Odocoileus and one Rangifer, and all species are restricted except three Odocoileus, one Rangifer and one Mazama. Brazilian records of Odoicoleus virginianus E. A. W. von Zimmermann, 1780 in Brazil are scarce, centred in Amapá state and are listed in Mendes-Oliveira et al. (Check List, 2011).
CETACEA
(42/)101 spp. worldwide in 14 families (ASM Mammal Diversity), being four Mysticeti (marine) and 10 Odontoceti (marine and freshwater), the two higher clades of this order.
MYSTICETI
Four families and (6/)15 spp.: Balaenidae (2/4, Balaena and Eubalanea, northern Hemisphere-3 and southern Hemisphere-1), Cetotheriidae (Caperea marginata Gray, 1846, southern Oceans), Balaenopteridae (2/9, Balaeonoptera and Megaptera) and Eschrichtiidae (Eschrichtius robustus Lilljeborg, 1861, Korea and Japan to NW Mexico).
Six species are not cited for Brazil, in Balaena (1), Eubalaena (2), Caparea (1), Eschrichtius (1) and Balaeonoptera (1, B. ricei Rosel et al., 2021, known only from Gulf of Mexico). Eight species no breeds in Brazil, in Balaena (1), Eubalaena (2), Caparea (1), Eschrichtius (1) and Balaeonoptera (3).
7 Mysticeti has consistent records of reproduction in Brazil (Sociedade Brasileira de Mastozoologia, EXCEL, 2023): Balaenoptera borealis Lesson, 1828 (Sei whale, ICMBio), B. bonaerensis Burmeister, 1867 (Antarctic minke whale, ICMBio), B. edeni Anderson, 1879 (Bryde's whale, ICMBio), B. physalus L., 1758 (Fin whale, Glienke, D., Dissertation, 2021), B. acutorostrata Lacépède, 1804 (Common minke whale, ICMBio), Megaptera novaeangliae Gray, 1846 (humpback whale, Morete, ME et al., LAJAM, 2022) and Eubalaena australis Desmoulins, 1822 (southern right whale, Seyboth, E. et al., Sci Rep, 2016).
BRAZILIAN MISTYCETI ADULTS IN SCALE
For two Mysticeti species recorded in Brazil, there is no confirmed record of reproduction of B. musculus L., 1758 (Blue whale, ICMBio, scarce records in Brazil: 1948, 1962, 1965, 1992 and the recent in 2020, ICMBIO, 2011), and B. omurai Wada, Oishi & Yamada, 2003, known physically only by a 4.16 m female calf found stranded in Pecem beach, Ceará state, in 2010 (Wiki | Cipriano-Souza, A. et al., Marine Mammal Science, 2016), and by vocal records in St. Paul and St. Peter Rocks (Moreira, SC et al., Journal of Mammology, 2020).
For blue whales in Brazil, see G1/2020 (Jornal Nacional, 2020), and G1/2019 (TV Tribuna, 2019). For the deepest dive from blue-whale in literature, see Relatório PMC (SEE), including the first monitoring data on the migration of the blue whale in the South Atlantic, and the indication of 7 visual records of the species off the coast of Brazil. Three records of mother-calf pairs was made in Ceará and Espirito Santo states in 2014 (Rocha et al., Marine Biodiversity Records, 2019).
ODONTOCETI
(36/)86 spp. in 10 families worldwide. 5 of these are fully nationally disjuncts except two simultaneously in Brazil: Lipotidae (1/1, China, extinct), Platanistidae (2/2, South Asian river dolphins, Pakistan, India, Nepal and Bangladesh), Iniidae (1/4, Amazon river dolphins, northern South America), Pontoporiidae (1/1, La Plata dolphins, SE Brazil to NE Argentina), Monodontidae (2/2, belugas and narwhals, Actic waters). These five families has 3 spp. in New World unknown in Brazil: Inia boliviensis (Bolivia), Phocoena sinus (NW Mexico), and P. phocoena (northern Hemisphere)
Five are widely distributed: Physeteridae (1/1, Physeter macrocephalus L., 1758, widely in seas, known in Brazil), Kogiidae (1/2, widely in seas, both known in Brazil/NCBI), Ziphiidae (6/22, Berardius and Hyperoodon two species each, one in each Hemisphere; Indopacetus and Tasmacetus are both monotypic and scattered highly rare in southern waters, and are not recorded in Brazil; Ziphius cavirostris Cuvier, 1823 widely in all oceans; Mesoplodon-16, widely in world seas, 5 of them cited for Brazil), Phocoenidae (3/8, Neophocaena three spp. from Persian Gulf up to Taiwan and rivers of China, Phocoenoides monotypic from northern Pacific from Japan to NW Mexico, these overlapping in Japan, and Phocoena-4, northern and southern Hemispheres one each, one in NW Mexico, and one around southern South America up to Ecuador and Brazil) and Delphinidae (17/37, oceanic dolphins, widely in world seas, detailed above).
At Delphinidae, 16 spp. in six genera do not occur in Brazil: Cephalorhynchus hectori (New Zealand), C. eutropia (Chile to Tierra del Fuego), C. heavisidii (Angola to South Africa), Tursiops aduncus (South Africa to New Caledonia and Japan), Lissodelphis borealis (Japan to NW Mexico in northern Hemisphere), Lagenorhynchus obliquidens (China to NW Mexico in northern Hemisphere), L. acutus (NE USA to Barents SEA), L. albirostris (NE USA to Barents SEA), L. cruciger (southern Ocean), L. obscurus (widely but scattered in southern Hemisphere), Sousa (4, Mauritania to Gabon, South Africa to New Caledonia and China, unknown in Brazil) and Orcaella (2, India to Australia, unknown in Brazil). All New World genera occur in Brazil, and 17 off 24 spp. in continent.
Ten Mesoplodon do not occur in Brazil: M. eueu (South Africa, Australia and New Zealand), M. bowdoini (Australia and New Zealand), M. ginkgodens (tropical Indo-Pacific, in New World from California to Chile), M. traversii (New Zealand and Chile), M. hotaula (scattered from Seycheles, Maldives, Sri Lanka, India, Taiwan, Japan, Australia, New Zealand, Hawaii and NW Mexico), M. carlhubbsi and M. stejnegeri (northern Pacific), M. perrini (off California), M. peruvianus (Mexico to Peru) and M. bidens (northern Atlantic).
Rare species in Brazil includes the northemost record of four spp.: Phocoena dioptrica, Mesoplodon layardii, Cephalorhynchus commersonii and Lagenorhynchus australis (Pinedo (Aquatic Mammals, 2002), all in Rio Grande do Sul coast. Relevant records of sperm whales in Brazil includes 40 individuals at 300 km from the coast (G1 | Conexão Planeta)in São Paulo state. Records of Cetacea and Sirenia stranded in Brazil since 1980 is available in Simmam (ICMBIO).
PERISSODACTYLA
(3:6/)18 spp. worldwide (ASM Mammal Diversity), only Tapiridae (1/4) living in New World, formed by Tapirus indicus Desmarest, 1819 (Indonesia, Malaysia, Myanmar, and Thailand), T. bairdii Gill, 1865 (Mexico, America Central and NW Colombia), T. terrestris L. 1758 (over South America except Chile and Uruguay), and T. pinchaque Roulin, 1829 (mountains in C to S Colombia, Ecuador and N Peru). Highest diversity in Colombia (3), Ecuador (2) and Peru (2).
▉ LAST UPDATE OF CRANIATA IN JANUARY/FEBRUARY 2026
LAST UPDATES
2026
Feb 17, 2026 ‣ numerical updates and textual corrections were made in Elasmobranchii (Eschmeyer's Catalog | CTFB), Actinopterii (Eschmeyer's Catalog | CTFB), Amphibia (Amphibian Species of the World 6.2), Testudines (Repfocus/Turtles), Aves (Catalogue of Life) and Mammals (ASM Mammals).
These change make the numbers of living and valid taxa as: Brazil from
(3,498:27,457/)127,412 spp. to (3,499:27,509/)127,776 spp., and the World from
(8,144:182,478/)1,595,117 spp. to (8,139:182,551/)1,596,517 spp.
Feb 17, 2026 ‣ review of Placozoa, Gnathostomulida, Micrognathozoa, Orthonectida, Phoronida, Loricifera, Phoronida, Myxini, Holocephali, Petromyzonti, Cladistii, Dipneusti, Coelacanthi, Rhynchocephalia, and Crocodylia clades of Craniata, with editorial adjustments but no numerical changes.
Feb 16, 2026 ‣ numerical updates and textual corrections were made in Ctenophora (Catalogue of Life), Porifera (Catalogue of Life | CTFB), Xenacoelomorpha (Catalogue of Life), Chaetognatha (Catalogue of Life | CTFB), Dicyemida (Catalogue of Life), Syndermata (Catalogue of Life | Catalogue of Life | CTFB | CTFB), Gastrotricha (Catalogue of Life), Entoprocta (Catalogue of Life | IZ, 2025), Nemertea (Catalogue of Life) — with the validation of a new canonical lineage within Nemertea, Arhynchocoela, a monotypic lineage endemic to New Zealand, the number of canonical lineages is increased to 145, Brachiopoda (Brachiopoda Database), Bryozoa (Catalogue of Life | CTFB), Kinorhyncha (Catalogue of Life), Priapulida (Catalogue of Life), Nematomorpha (Catalogue of Life), Nematoda (Catalogue of Life | CTFB), Echinodermata (Catalogue of Life | CTFB), Hemichordata (Catalogue of Life | CTFB | Plos One, 2016), Epigonichthys from World (Copepedia | Catalogue of Life) and Branchiostoma for Brazil (CTFB) in Cephalochordata, and Tunicata (Catalogue of Life | CTFB | CTFB).
These change make the numbers of living and valid taxa as: Brazil from
(3,491:27,427/)127,390 spp. to (3,498:27,457/)127,412 spp., and the World from
(7,983:181,116/)1,588,863 spp. to (8,144:182,478/)1,595,117 spp.
Feb 15, 2026 ‣ numerical updates and textual corrections were made in Annelida (Cataogue of Life | CTFB), with the inclusion of Questidae in Orbiniidae, thus reducing the number of canonical Metazoa lineages from 145 to 144.
These change make the numbers of living and valid taxa as: Brazil from
(3,493:27,429/)127,413 spp. to (3,491:27,427/)127,390 spp., and the World from
(7,975:180,867/)1,587,307 spp. to (7,983:181,116/)1,588,863 spp.
Feb 14, 2026 ‣ numerical updates and textual corrections were made in Cnidaria (Catalogue of Life | CTFB), Platyhelminthes (Catalogue of Life | CTFB/Platyhelminthes) and Mollusca (Catalogue of Life | CTFB).
These change make the numbers of living and valid taxa as: Brazil from
(3,495:27,334/)127,085 spp. to (3,493:27,429/)127,413 spp., and the World from
(7,732:177,851/)1,568,276 spp. to (7,975:180,867/)1,587,307 spp.
Feb 13, 2026 ‣ numerical updates and textual corrections were made in Insecta (Catalogue of Life | CTFB/Insecta
| Irish et al., Zoologischer Anzeiger, 2025 | several small workers).
These change make the numbers as: Brazil from
(3,485:27,195/)127,309 spp. to (3,495:27,334/)127,085 spp., and the World from
(7,497:152,216/)1,548,157 spp. to (7,732:177,851/)1,568,276 spp.
Feb 12, 2026 ‣ numerical updates and textual corrections were made in Malacostraca (Catalogue of Life | CTFB | and several article-sources), Collembola (Catalogue of Life | CTFB), Protura (Catalogue of Life | CTFB) and Diplura (Catalogue of Life | Sendra, Insect Conservation and Diversity, 2021 | CTFB).
These change make the numbers as: Brazil from
(3,475:27,143/)127,080 spp. to (3,485:27,195/)127,309 spp., and the World from
(7,535:152,373/)1,548,020 spp. to (7,497:152,216/)1,548,157 spp.
Feb 07, 2026 ‣ numerical updates and textual corrections were made in Pycnogonida (Catalogue of Life | CTFB) and Oligostraca (Catalogue of Life | CTFB), Diplopoda (mainly on Catalogue of Life and numerous descriptions of new species in Brazil), Thecostraca (Catalogue of Life | CTFB), Copepoda (Catalogue of Life | CTFB), and Branchiopoda (Catalogue of Life | CTFB), and textual correction in Remipedia and Cephalocarida.
These change make the numbers as: Brazil from
(3,503:27,104/)127,057 spp. to (3,475:27,143/)127,080 spp., and the World from
(7,496:151,342/)1,542,821 spp. to (7,535:152,373/)1,548,020 spp.
Feb 02, 2026 ‣ numerical updates and textual corrections were made in Pauropoda (Catalogue of Life | CTFB) and Symphyla (Catalogue of Life).
These change make the numbers as: Brazil from
(3,503:27,104/)127,056 spp. to (3,503:27,104/)127,057 spp., and the World from
(7,496:151,331/)1,542,586 spp. to (7,496:151,342/)1,542,821 spp.
Jan 31, 2026 ‣ numerical updates and textual corrections were made in Arachnida after Catalogue of Life/Arachnida, CTFB/Arachnida, The Scorpion Files (SEE), and World Arachnida Catalog (SEE), and in Chilopoda after Catalogue of Life/Chilopoda, CTFB/Chilopoda and several article-sources.
These change make the numbers as: Brazil from
(3,495:27,117/)127,741 spp. to (3,503:27,104/)127,056 spp., and the World from
(7,426:150,437/)1,555,470 spp. to (7,496:151,331/)1,542,586 spp.
Jan 26, 2026 ‣ numerical updates and textual corrections were made in Tardigrada, Onychophora and Cycliophora after Catalogue of Life (EUT | HET | CYC) and CTFB (SEE), and updates in numbers of Colombia and Mexico (MX | CL).
These change make the numbers as: Brazil from
(3,495:27,117/)127,739 spp. to (3,495:27,117/)127,741 spp., and the World from
(7,424:150,422/)1,555,408 spp. to (7,426:150,437/)1,555,470 spp.
2025
May 10, 2025 ‣ numerical updates and textual corrections were made in Insecta, Amphibia, Squamata and Testudines. These change make the numbers as: Brazil from
(3,488:27,054/)127,365 spp. to (3,495:27,117/)127,739 spp., and the World from
(7,424:150,415/)1,555,512 spp. to (7,424:150,422/)1,555,408 spp.
May 05, 2025 ‣ we include the record of Craniiformea in Brazil, based on a collection made off the coast of Bahia in 1997 (Invertbase/Voos Marine Invertebrate Collection). These change make the numbers as: Brazil from
(3,487:27,053/)127,364 spp. to (3,488:27,054/)127,365 spp., and the World from
(7,424:150,415/)1,555,512 spp. to (7,424:150,415/)1,555,512 spp.
May 01, 2025 ‣ numerical updates and textual corrections were made in Myxine, Petromyzonti, Elasmobranchii, Actinopteri, Holocephali, Cladistii, and Aves. These changes make the numbers as: Brazil from
(3,549:27,081/)127,643 spp. to (3,487:27,053/)127,364 spp., and the World from
(7,502:150,645/)1,556,450 spp. to (7,424:150,415/)1,555,512 spp.
April 27, 2024 ‣ update in Brachiopoda species, in Onychophora species and genera, and huge updates in Phoronida, with revision of species worldwide, including the reclassification of the group and the reduction from two to one canonical lineages, along with a decrease in the total number of recognized species worldwide from 146 to 145. These changes make the numbers as: Brazil from
(3,549:27,082/)127,650 spp. to (3,549:27,081/)127,643 spp., and the World from
(7,502:150,642/)1,556,455 spp. to (7,502:150,645/)1,556,450 spp.
April 26, 2024 ‣ thorough and comprehensive revision and update of Syndermata, Nemertea, Gastrotricha, Tardigrada, Kinorhyncha, Nematomorpha, Malacostraca and Tunicata, including nomenclatural, numerical, orthographic, and taxonomic changes after CTFB and Catalogue of Life (SEE), changes in Chaetogantha, Entoporcta, Bryozoa and Hemichordata, including the reclassification of these three phylla and the reduction from three to two canonical lineages in first, four to 2 in the second, four to three in third, and three to two in the last, along with a decrease in the total number of recognized species worldwide from 151 to 146. These changes make the numbers as: Brazil from
(3,511:26,985/)127,263 spp. to (3,549:27,082/)127,650 spp., and the World from
(7,295:149,030/)1,548,863 spp. to (7,502:150,642/)1,556,455 spp.
April 25, 2024 ‣ updates in Nematoda: revision of phylum numbers worldwide (Catalogue of Life) and in Brazil (CTFB/SEE), including the reclassification of the group and the reduction from three to two canonical lineages (Dorylaimea was removed), along with a decrease in the total number of recognized species worldwide from 152 to 151. These changes make the numbers as: Brazil from
(3,491:26,635/)127,121 spp. to (3,511:26,985/)127,263 spp., and the World from
(7,296:149,446/)1,553,547 spp. to (7,295:149,030/)1,548,863 spp.
April 17, 2024 ‣ thorough and comprehensive revision and update of Ctenophora, Cnidaria, Placozoa, Porifera, Xenacoelomorpha, Annelida, Mollusca and Nematoda, including numerical, orthographic, and taxonomic changes after CTFB and Catalogue of Life (SEE). These changes make the numbers as: Brazil from
(3,466:26,547/)126,745 spp. to (3,491:26,635/)127,121 spp., and the World from
(7,429:142,505/)1,555,380 spp. to (7,296:149,446/)1,553,547 spp.
April 13, 2025 ‣ numerical updates in Copepoda (taxonomic adjustments, with slight additions of families, genera, and species: CTFB | Alvarez, MPJ., Journal of Natural History, 1985 | Araújo & Neumann-Leitão, Annals of the Brazilian Academy of Sciences, 2015), Thecostraca (numerical and texonomic: CTFB | Chan, B.K.K. et al., ZJBS, 2021), Branchiopoda (slight additions of families, genera, and species: CTFB | Rogers, 2020 | Pessac et al., Zootaxa, 2011 / Fonseca et al., Wetlands, 2017) and Echinodermata (CTFB | Manso, C.L.C., Biota Neotropica, 2008 | Catalogue of Life). These changes make the numbers as: Brazil from
(3,437:26,476/)126,613 spp. to (3,466:26,547/)126,745 spp., and the World from
(7,405:141,827/)1,554,751 spp. to (7,429:142,505/)1,555,380 spp.
April 04, 2025 ‣ updates in Pauropoda numbers and taxonomy (SEE | SEE), Chilopoda numbers (437/3,327, SEE), Cephalochordata in Brazil (SEE | SEE | SEE | SEE | SEE | SEE, with a exclusion of two canonic lineages) and in numbers and phylogeny of Priapulida (SEE | SEE, with the additon of two canonic lineages: Meiopriapulus and Maccabeus). These changes make the numbers as: Brazil from (3,436:26,470/)126,600 spp. to (3,437:26,476/)126,613 spp., and the World from (7,401:141,725/)1,554,486 spp. to (7,405:141,827/)1,554,751 spp.
March 15, 2025 ‣ non numerical updates among orders of Copepoda (SEE) and notes for future updates among Haplotaxida (SEE).
March 01, 2025 ‣ updates in Uropygi in Brazil (Invertebrates Systematics, 2025), in World and in Brazil in Amblypygi, Solifugae (genera and species numbers), Pseudoscorpiona, Ricinulei, Palpigradi and Schizomida by World Arachnida Catalogue (SEE), world numbers for Araneae by WSC/Statistics (SEE), and additon of a new species in Micrognathozoa (SEE). These changes make the numbers as: Brazil from (3,436:26,470/)126,588 spp. to (3,436:26,470/)126,600 spp., and the World from (7,399:141,678/)1,553,069 to (7,401:141,725/)1,554,486 spp.
2024
December 24, 2024 ‣ huge updates in Coleoptera for Brazil (SEE), Araneae (SEE), Opliona (SEE), Solifugae (SEE), Scorpiona (SEE) and Thelyphonida (SEE). These changes make the numbers as: Brazil from (3,434:26,449/)126,628 spp. to (3,436:26,470/)126,588 spp., and the World from (7,396:141,602/)1,551,907 to (7,399:141,678/)1,553,069 spp.
December 23, 2024 ‣ major updates in the Pancrustacea group, with the inclusion of Brachiura and Mystacocarida in an expanded form of Ostracoda, namely here as Oligostraca, and the partition of Hexanauplia into Copepoda and Thecostraca (including Tantulocarida), based on Bernot, J.P. et al. (Molecular Biology and Evolution, 2023). This change does not alter the numbers of families, genera, or species, but modifies the arrangement of the canonical classes in Metazoa, with the numerical reduction of one group.
December 22, 2024 ‣ updates in Scalibregmatidae/Travisidae (SEE | SEE) and Aeolosomatidae/Hrabeiellidae (SEE). These changes make the numbers as: Brazil from (3,433:26,447/)126,621 spp. to (3,434:26,449/)126,628 spp., and the World from (7,395:141,601/)1,551,907 to (7,396:141,602/)1,551,907 spp.
December 17, 2024 ‣ huge, numeric, textual and estrutural updates in Platyhelminthes, and relisting of canonic lineages of Metazoa, including down of Haplopharyngida. These changes make the numbers as: Brazil from (3,427:26,451/)126,594 spp. to (3,433:26,447/)126,621 spp., and the World from (7,356:141,019/)1,552,159 to (7,395:141,601/)1,551,907 spp.
December 9, 2024 ‣ updates in Spionida (SEE) and Siboglinidae (SEE) in Annelida. These changes make the numbers as: Brazil from (3,424:26,445/)126,577 spp. to (3,427:26,451/)126,594 spp., and the World from (7,356:141,019/)1,552,159 to (7,356:141,019/)1,552,159 spp.
December 7, 2024 ‣ updates in Mammalia. These changes make the numbers as: Brazil from (3,426:26,444/)126,577 spp. to (3,424:26,445/)126,577 spp., and the World from (7,348:140,994/)1,551,052 to (7,356:141,019/)1,552,159 spp.
November 27, 2024 ‣ updates in Avialia and Squamata. These changes make the numbers as: Brazil from (3,425:26,448/)126,575 spp. to (3,426:26,444/)126,577 spp., and the World from (7,348:140,994/)1,551,052 to (7,356:141,009/)1,551,960 spp.
November 25, 2024 ‣ restructuring, optimization, and updating in Amphibia and Testudines, restructuring in Avialia, optimizations in Sphenodontia and Crocodylia. These changes make the numbers as: Brazil from (3,422:26,444/)126,590 spp. to (3,425:26,448/)126,575 spp., and the World from (7,344:140,984/)1,550,866 spp. to (7,348:140,994/)1,551,052 spp.
November 16, 2024 ‣ inclusion of Micrognathozoa in the list of Brazil, with consequent updates in various parts of the text (Coppo, G. et al., Peerj, 2023). Huge numerical and strutural updates in Fish clades, after Eschmeyer's CF, Fish Base, and Intreasures, all in Nov 16, 2024. Simple numerical updates in Hydrozoa (- 3 spp.), Cheilostomata in Bryozoa (10,000 to 4,921 spp.) and Cycliophora (2 to 3 spp.). Huge taxonomic updates in Placozoa, with reduction of non Brazilian canonic lineages and expansion of family, genera and species in this phyllum (Frontiers, 2023). Textual corrections and optimization in Porifera (SEE), Myxozoa, Acoela, Gnathostomulida, Priapulida, and many other groups. Addition of a endemic family for Peru, Atamatamidae (Trematoda, SEE). These changes make the numbers as: Brazil from (3,409:26,416/)126,358 spp. to (3,422:26,444/)126,590 spp., and the World from (7,334:140,921/)1,555,398 spp. to (7,344:140,984/)1,550,866 spp.
April 29, 2024 ‣ updates in Squamata (news), Ricinulei (notes about possibly new genera), notes from Brazilian scorpions, new remarkable data from Scutigeridae in Chilopoda and Mostrilloidea at Copepoda; huge updates in numbers of Tunicata (Rocha, R.M. et al., Zoologia, 2024), including a synonimization of Thaliacea under Ascidiacea, rearranged canonic lineages from Metazoa by reduction of 1 unit and altering the numbers of the phylum and the total Metazoa; updates in Scalibregmatidae, with 8 new species, in Pseudoscalibregma (3, SEE), Scalibregma (8, SEE), and Oligobregma (3, SEE). United, these changes the numbers as: Brazil from (3,410:26,411/)126,308 spp. to (3,409:26,416/)126,358 spp., and the World from (7,337:140,921/)1,555,228 spp. to (7,334:140,921/)1,555,398 spp.
21∙01∙2024 ▸ updates in Scorpiona, based on The Scorpion Files.
20∙01∙2024 ▸ notes on Placozoa, including possible requalification of canonic lineages at Metazoa (SEE); structural revision in Isopoda, Amphipoda and Decapoda after CTFB (SEE).
16∙01∙2024 ▸ exclusion of the Maxillopoda group due to its poliphyleticity, and the recognition of 3 new classes in Arthropoda: Mystacocarida, Ichthyostraca and Hexanauplia (Wikipedia); exclusion of Merostomata as a class, and its recognition as an order of Arachnida, according to Balasteros et al. (SEE); huge numeric updates at Copepoda.
14∙01∙2024 ▸ huge updates in Decapoda for several small sources, and at Insecta, Collembola, Diplura and Protura after Rafael et al. (BOOK)
10∙01∙2024 ▸ huge updates in Hemichordata and the exclusion of Hirudinidae family from Brazil.
07∙01∙2024 ▸ huge numerical updates in Arachnida, Insecta and Mammals at high precision counting by available data. After corrections, Insecta wins 70 families, 426 genera and 847 spp., and Arachnida wins 112 families, 559 genera and 1,660 spp. in Brazil. Brazilian diversity went from (3,221:25,596/)121,461 to (3,403:26,579/)123,993.
04∙01∙2024 ▸ updates among Cyprinidae (SEE); huge updates and organization in Serpentes, Insecta, Arachnida and mammals, and rearrangement of various numbers, and inclusion of several references about Mexico; updates in Lepidochelys kempii (NOAA).
2023
19∙12∙2023 ▸ several numerical, textual and spelling corrections in Arachnida and Insecta.
29∙12∙2023 ▸ number updates in Brazilian Maxillopoda, Branchiopoda and Malacostraca, mainly by CTFB search (SEE).
27∙12∙2023 ▸ updates among Elasmobranchii in Brazil and Mexico (FishBase), and new data about Chilopoda in Mexico (SEE) and Brazil (SEE).
19∙12∙2023 ▸ addition of notes for Conocyemidae (SEE and SEE) and Pelmatosphaeridae (SEE), nanoparasitics Mesozoa.
18∙12∙2023 ▸ addition of notes for marine Brazilian Nematoda (SEE), world marine Tardigrada (SEE), Siboglinidae (SEE) and Sinelobus (SEE).
18∙12∙2023 ▸ huge updates in Brazilian Mollusca, by F.M. Machado et al. (Zoologia, 2023).
18∙12∙2023 ▸ addition of notes for Onychophora, with 20 more species (SEE), Leopardus emilieae (Felidae, SEE), freshwater Cumacea (SEE), Leuconidae (Cumacea, SEE).
18∙12∙2023 ▸ addition of notes for mammals (in Proechimys), in Xyloplax at Asteroideae (SEE), in Scolopendromorpha (SEE) and Araneae/Telemidae (SEE).
04∙12∙2023 ▸ addition of data on the first occurrence of Lingulidae (Brachiopoda) in Brazil (SEE).
12∙11∙2023 ▸ huge updates on Cephalopoda (mainly by SEE), and corrections in Gastropoda numbers.
10∙11∙2023 ▸ updates on Loricifera, with the inclusion of the first species described for Brazil, Scaberiloricus samba (SEE).
06∙11∙2023 ▸ updates in Avialia, where we now consider only breeding species as full natives (SEE).
04∙11∙2023 ▸ several updates and inclusion of records of a new phylla from Brazil: Gnathostomulida (SEE), and new generic and familiar records for Kinorhycnha (SEE).
03∙11∙2023 ▸ updates in numbers of Acanthocepala for Brazil (SEE) and World (SEE).
14∙01∙2024 ▸ huge updates in Decapoda for several small sources, and at Insecta, Collembola, Diplura and Protura after Rafael et al. (BOOK)
10∙01∙2024 ▸ huge updates in Hemichordata and the exclusion of Hirudinidae family from Brazil.
07∙01∙2024 ▸ huge numerical updates in Arachnida, Insecta and Mammals at high precision counting by available data. After corrections, Insecta wins 70 families, 426 genera and 847 spp., and Arachnida wins 112 families, 559 genera and 1,660 spp. in Brazil. Brazilian diversity went from (3,221:25,596/)121,461 to (3,403:26,579/)123,993.
04∙01∙2024 ▸ updates among Cyprinidae (SEE); huge updates and organization in Serpentes, Insecta, Arachnida and mammals, and rearrangement of various numbers, and inclusion of several references about Mexico; updates in Lepidochelys kempii (NOAA).
2023
19∙12∙2023 ▸ several numerical, textual and spelling corrections in Arachnida and Insecta.
29∙12∙2023 ▸ number updates in Brazilian Maxillopoda, Branchiopoda and Malacostraca, mainly by CTFB search (SEE).
27∙12∙2023 ▸ updates among Elasmobranchii in Brazil and Mexico (FishBase), and new data about Chilopoda in Mexico (SEE) and Brazil (SEE).
19∙12∙2023 ▸ addition of notes for Conocyemidae (SEE and SEE) and Pelmatosphaeridae (SEE), nanoparasitics Mesozoa.
18∙12∙2023 ▸ addition of notes for marine Brazilian Nematoda (SEE), world marine Tardigrada (SEE), Siboglinidae (SEE) and Sinelobus (SEE).
18∙12∙2023 ▸ huge updates in Brazilian Mollusca, by F.M. Machado et al. (Zoologia, 2023).
18∙12∙2023 ▸ addition of notes for Onychophora, with 20 more species (SEE), Leopardus emilieae (Felidae, SEE), freshwater Cumacea (SEE), Leuconidae (Cumacea, SEE).
18∙12∙2023 ▸ addition of notes for mammals (in Proechimys), in Xyloplax at Asteroideae (SEE), in Scolopendromorpha (SEE) and Araneae/Telemidae (SEE).
04∙12∙2023 ▸ addition of data on the first occurrence of Lingulidae (Brachiopoda) in Brazil (SEE).
12∙11∙2023 ▸ huge updates on Cephalopoda (mainly by SEE), and corrections in Gastropoda numbers.
10∙11∙2023 ▸ updates on Loricifera, with the inclusion of the first species described for Brazil, Scaberiloricus samba (SEE).
06∙11∙2023 ▸ updates in Avialia, where we now consider only breeding species as full natives (SEE).
04∙11∙2023 ▸ several updates and inclusion of records of a new phylla from Brazil: Gnathostomulida (SEE), and new generic and familiar records for Kinorhycnha (SEE).
03∙11∙2023 ▸ huge updates in Priapulomorpha.
03∙11∙2023 ▸ add lineage, Octocorallia distinct of Hexacorallia in Cnidaria, rejecting the broad circumscription of Anthozoa, and updates of the numbers of Octocorallia (SEE).
21∙10∙2023 ▸ addition of the Cephalodiscus record in Brazil, based on collections made in Rio Grande do Sul (REVIZEE, 2004; REVIZEE, 2008).
16∙10∙2023 ▸ huge updates in Arachnida (many orders), Chilopoda, Scalibregmatidae (SEE) and over Annelida), with minor text corrections and some notes at mammals.
06∙09∙2023 ▸ huge updates in Schizomida, Solifugae, Araneae (these Arachnida), Chilopoda, Diplopoda, Insecta, Symphyla, Pauropoda and Pentastomida.
02∙09∙2023 ▸ huge updates in Gnathostomulida.
28∙08∙2023 ▸ update data distribution of Gnosonesimida in Cuba (SEE) and detailing in Orthonectida.
25∙07∙2023 ▸ update data from Mexico based on references cited in Bousquets et al. (Conabio, 2000).
24∙07∙2023 ▸ additional notes in mexican amphibians and reptiles (ZooKeys, 2023), and inclusion of Ophidion holbrookii (Ophidiidae, Zootaxa) in Brazilian marine fishes.
20∙07∙2023 ▸ updates in Amblipigy.
15∙07∙2023 ▸ updates in Loricifera in South America.
13∙07∙2023 ▸ updates in Ammotrechidae, Diplopauropodidae, Echinoidea orders and Copepoda orders.
06∙07∙2023 ▸ updates in Diplopoda and social wasps, and severall small corrections.
03∙07∙2023 ▸ updates in Nereidae in Annelida.
27∙06∙2023 ▸ updates in Gnosonesimida, Haplopharyngida (Platyhelminthes) and Monoplacophora (Mollusca).
19∙06∙2023 ▸ updates in Kinnorhyncha.
10∙06∙2023 ▸ updates in aquatic Clitellata (Annelidae), Rhabdocoela in Platyhelminthes, and data in Colombian Collembola.
09∙06∙2023 ▸ inclusion of links about Mammals/Carnivorous, Arachnida/Scorpiones and Mollusca/Heterobranchia.
03∙06∙2023 ▸ updates in Siboglinidae (Annelidae).
09∙05∙2023 ▸ updates in Porifera, pisces and Amphibia numbers.
09∙05∙2023 ▸ updates in Gymnophiona numbers, with Colombia suprasing Brazil in species diversity.
02∙05∙2023 ▸ updates on taxonomy of gastropoda, cephalopoda and bivalvia, and new numbers in Brachiopoda (Terebratullida).
20∙03∙2023 ▸ huge updates in Annelida/Clitellata and Branchiopoda.
04∙01∙2023 ▸ a huge update in many groups.
06∙08∙2022 ▸ a huge update in Insecta numbers and text.
06∙08∙2022 ▸ huge updates in Annelida, with inclusion of Echiura and Sipuncula inside the former, and other providences.
24∙07∙2022 ▸ a major review and data optimization, inclusion of references and textual corrections.
11∙07∙2022 ▸ updates in Carideae, with the exclusion of Kakaducarididae (now inside Palaeomonidae).
11∙07∙2022 ▸ updates in Onychophora (phylogeny) and Rotifera (data from Mexico).
10∙07∙2022 ▸ recognition of Nemamyxine kreffti (Myxinidae) in Brazil.
27∙06∙2022 ▸ exclusion of Cyptogeobiidae as endemic Opiliones family in Brazil
27∙06∙2022 ▸ inclusion of data from Allokoenenia in Brazil.
13∙06∙2022 ▸ updates in many numbers in almost all classes.
20∙03∙2022 ▸ inclusion of data on the new Jurasaidae family of Coleoptera, endemic to Brazil.
11∙02∙2022 ▸ updates in Rotifera (new family added in numbers) and changes in Hypoechinorhynchidae name in Acanthocephala.
2021
04∙12∙2021 ▸ additional data of albinism.
26∙11∙2021 ▸ updates in Polypodiozoa and bioluminescent Clitellata and Mollusca.
20∙11∙2021 ▸ updates in lobsters (Decapoda).
20∙11∙2021 ▸ updates in Schizomida.
18∙11∙2021 ▸ some data in birds and mexican endemic marine fihes.
18∙11∙2021 ▸ addition of informations about Lama (Camelidae).
18∙11∙2021 ▸ additon of south american number of bird species, and Capitonidae as Mexican outsider.
12∙11∙2021 ▸ spell checking, text optimization and light restructuring.
ANNEX ‣ main bibliographical references in zoology.
ZOOTAXA
ZOOKEYS
EUROPEAN JOURNAL OF TAXONOMY
PAPÉIS AVULSOS DE ZOOLOGIA
ZOOSYSTEMA
IHERINGIA
NAUPLIUS
DIVERSITY
CHECKLIST
SUBTERRANEAN BIOLOGY
VERTEBRATE ZOOLOGY
PEERJ - ZOOLOGY
ICHTHYOLOGY & HERPETOLOGY
MARINE BIODIVERSITY
REVISTA BRASILEIRA DE ZOOLOGIA
ORGANISMS DIVERSITY AND EVOLUTION
ZOOSYSTEMATICA ROSSICA
ZOOLOGISCHER ANZEIGER
AUSTRAL ENTOMOLOGY
BULLETIN OF THE SOCIETY OF SYSTEMATIC BIOLOGISTS
ACTA PARASITOLOGICA
JOURNAL OF NATURAL HISTORY
ACTA OECOLOGICA
TAXONOMY
INSECTS
REVISTA BRASILEIRA DE ENTOMOLOGIA
ANNALES ZOOLOGICI FENNICI
TRANSACTIONS OF THE AMERICAN ENTOMOLOGICAL SOCIETY
ARTHROPOD SYSTEMATICS & PHYLOGENY
DUGESIANA
INSECT SYSTEMATICS AND DIVERSITY
CLADISTICS
ZOOLOGICAL JOURNAL OF LINNEAN SOCIETY
INVERTEBRATE SYSTEMATICS
NEOTROPICAL BIOLOGY AND CONSERVATION
TERESINA, PIAUÍ, BRAZIL